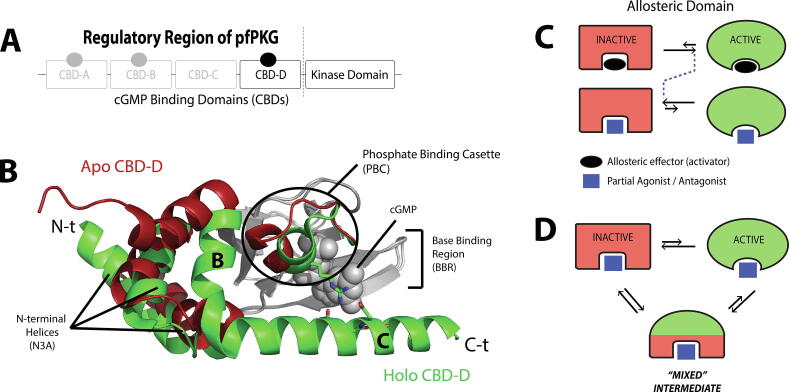
Fig. 1.
Inhibition by allosteric partial agonists through perturbation of conformational equilibria. (A) An example of domain organization in a cyclic-nucleotide activated enzyme, i.e. the cGMP-dependent protein kinase (PKG) of Plasmodium falciparum. In this example, the regulatory and catalytic regions are in the same polypeptide chain, and thus the C-terminal cyclic-nucleotide binding domain (CBD) is directly linked to the catalytic region. (B) An example of the CBD structural architecture in the apo inactive state (red) and the holo active state (green). The grey region indicates the largely invariant β-subdomain. The N-terminal helices (N3A), the C-terminal helices (“B” and “C”), and the phosphate binding cassette (PBC) undergo significant structural changes upon binding to the cGMP effector. (C) When an allosteric domain equilibrates between inactive and active states, binding of its endogenous allosteric effector (activator) typically shifts the equilibrium to the active state. A partial agonist or antagonist can either shift the equilibrium towards the inactive state, or sample an ensemble of conformations where an additional “mixed” intermediate state is sampled, as shown in panel (D). The mixed nature of this intermediate is manifested in its ability to resemble more closely the active state in some regions, and the inactive state in other regions, as depicted by the green/red color pattern. The figure was originally published in the Journal of Biological Chemistry. Byun JA, Van K, Huang J, Henning P, Franz E, Akimoto M, et al. Mechanism of allosteric inhibition in the Plasmodium falciparum cGMP-dependent protein kinase. J. Biol. Chem. 2020;295:8480–91. © the American Society for Biochemistry and Molecular Biology. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)