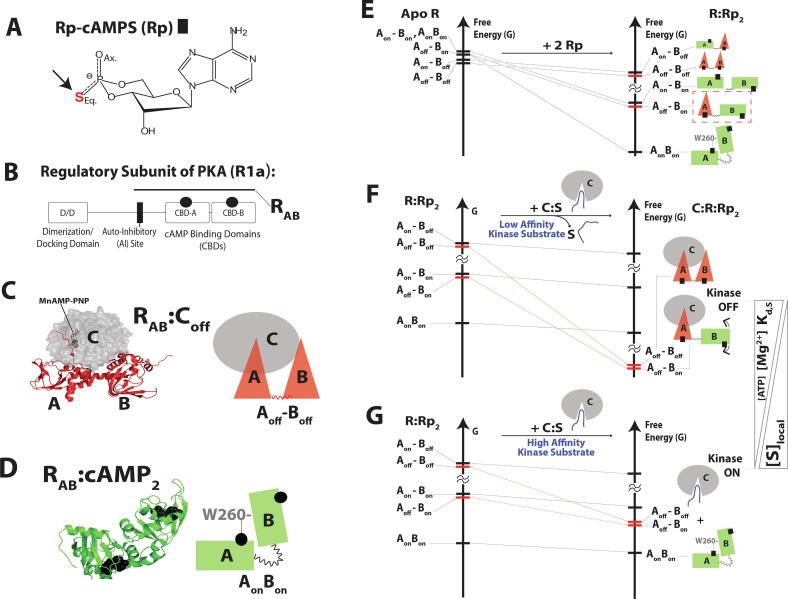
Fig. 3.
The multidomain PKA regulatory subunit bound to an allosteric inhibitor, Rp-cAMPS, samples an excited “mixed” state (Aoff-Bon) that drives allosteric pluripotency. (A) Structure of Rp-cAMPS. (B) Domain organization of the regulatory subunit. (C) In the absence of cAMP, the regulatory subunit (R) inhibits the catalytic subunit (C). The inhibitory site linked to CBD-A docks into the active site of the C-subunit, and is further stabilized by MgATP (or MnAMP-PNP, which is a non-hydrolyzable ATP analogue). (D) When cAMP binds to each of the CBDs, conformational changes occur that allow the C-subunit to be released. The inter-CBD interaction through W260 stabilizes the “on” conformations of the CBDs. (E) The free-energy hierarchy of apo R and free energy changes upon binding of Rp. The AonBon becomes the ground state, and the inhibition-competent “mixed” state (Aoff-Bon) is one of the excited states (red dashed box). (F) When the C-subunit is added to R:Rp2 in the presence of high [MgATP], the inhibition-competent states (red bars) are stabilized and become the ground states. (G) When the C-subunit is added to R:Rp2 in the absence of MgATP, the R:C interaction is less stable, and the inhibition-competent states remain excited. Since the ground state conformer exhibits low affinity for PKA C, the kinase function is activated. Figures are adapted from Byun JA, Akimoto M, VanSchouwen B, Lazarou TS, Taylor SS, Melacini G. Allosteric pluripotency as revealed by protein kinase A. Sci Adv 2020;6:eabb1250. Reprinted with permission from AAAS. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)