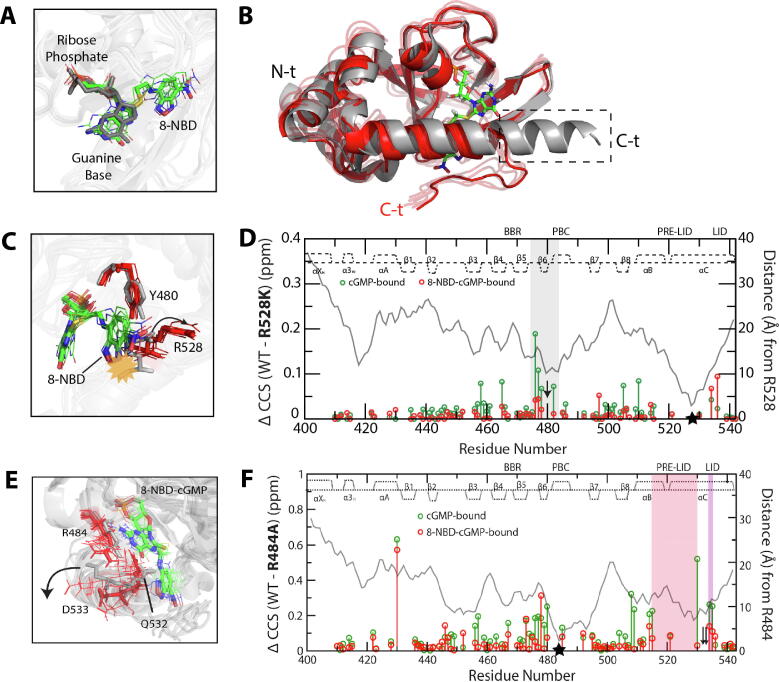
Fig. 4.
Effect of 8-NBD-cGMP bound to pfPKG CBD-D on C-terminal helix interactions necessary for kinase activation. (A) Overlay of the bound ligands from cGMP-bound CBD-D, and from representative structures of 8-NBD-cGMP-bound CBD-D generated from MD simulations. The syn orientation of cGMP is preserved in 8-NBD-cGMP. (B) Aligned representative structures of cGMP-bound CBD-D (grey) and 8-NBD-cGMP-bound CBD-D (red) generated from the MD simulations. The dashed box indicates the C-terminal lid region that becomes disordered in the 8-NBD-cGMP-bound CBD-D, as schematically shown in Fig. 2B. (C) Similar to panel (B), but zoomed into the Y480-R528 region. The yellow starburst indicates the steric clash of the 8-NBD substituent with the R528 side chain in the active structure, and the arrow indicates the structural shift of the R528 side chain upon binding of 8-NBD-cGMP. (D) WT-versus-R528K chemical shift differences for cGMP-bound (green) and 8-NBD-cGMP-bound (red) CBD-D. The distance from R528 (as measured in the cGMP-bound structure) is shown as a grey line, and the secondary structure in the cGMP-bound CBD-D is indicated at the top of the plot. The mutation site is indicated by a black star, and the grey highlight indicates residues near Y480 (black arrow), where perturbations induced by the mutation in the cGMP-bound complex are lost when cGMP is replaced by 8-NBD-cGMP. (E) Similar to panel (C), but zoomed into the capping-triad region (i.e. R484, Q532, D533) to highlight the shift of the D533 side chain (arrow) upon binding of 8-NBD-cGMP. (F) Similar to panel (D), but for the R484A mutant of CBD-D. Pre-lid and lid residues are indicated by pink and purple highlights, respectively, and capping-triad residues Q532 and D533 by black arrows. The figures were adapted with permission and were originally published in the Journal of Biological Chemistry. Byun JA, Van K, Huang J, Henning P, Franz E, Akimoto M, et al. Mechanism of allosteric inhibition in the Plasmodium falciparum cGMP-dependent protein kinase. J. Biol. Chem. 2020;295:8480–91. © the American Society for Biochemistry and Molecular Biology. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)