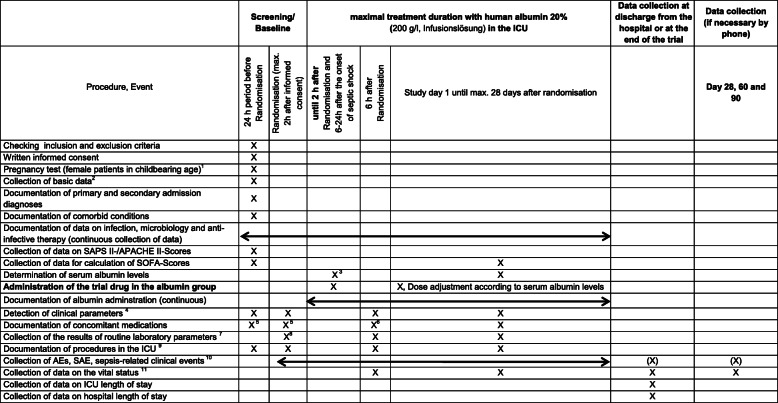
Fig. 2.
Schedule of the trial visits and study procedures. 1After obtaining informed consent. 2Basic data: sex, age, weight, height, time of hospital admission, time of intensive care unit (ICU) admission, type of admission, referring facility prior to ICU admission, etc. from the 24-h period before randomization (data from ICU or normal ward). 3Albumin group: determination of serum albumin concentration before administration of the starting dose of the trial drug. 4Body temperature, respiratory rate, and haemodynamic parameters. 5Catecholamines, inotropic agents, diuretics, volume replacement therapy, including blood transfusion, adjunctive sepsis therapy. 6Recording of concomitant medication with catecholamines and inotropes at the corresponding time point (+/− 1 h); recording of concomitant medication with diuretics, volume therapeutics including transfusion therapy, adjunctive sepsis therapy of the previous 6 h. 7Haemoglobin, creatinine, bilirubin, C-reactive protein, procalcitonin, leucocytes, platelets, lactate, arterial blood gas analysis. 824-h time period prior to randomization. 9Procedures, mechanical ventilation, haemodynamic monitoring. 10If adverse events (AEs) or serious adverse events (SAEs) are still “ongoing” after the end of treatment with the trial drug, observation will continue until the end of data collection (day 90). 11Data on the vital status (alive/dead), if applicable date of death and cause of death, residence after discharge, if applicable, recording of “End of Life” decisions