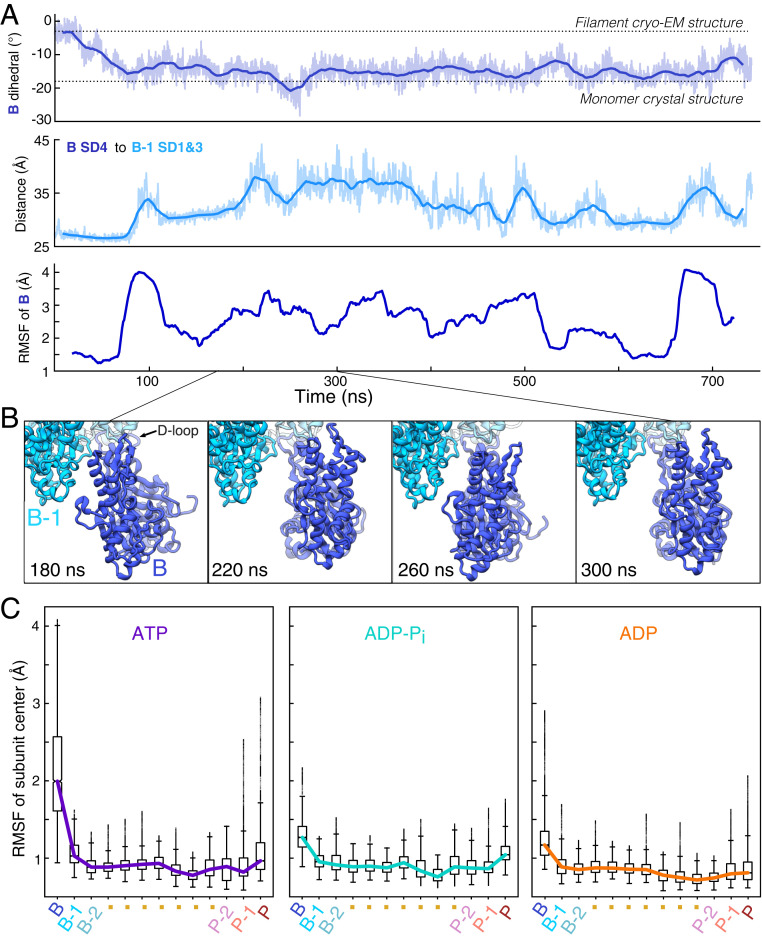
Fig. 2.
The transition of the ATP barbed end subunit to a monomeric dihedral angle is associated with loss of lateral subunit contacts, while remaining tethered to the barbed end by its D-loop. (A) Time course of changes during a 742-ns MD simulation of an ATP-actin filament. The Top and Middle figures show raw data (light) and 20-ns moving averages (dark). (Top) The dihedral angle of barbed end subunit B spontaneously increases as the subunit transitions to a monomeric conformation. (Middle) The distance between centers of mass of subdomain 4 of subunit B and subdomains 1 and 2 of subunit B-1 increase as subunit B transitions to a monomer-like structure. (Bottom) The RMSF in 40-ns windows center of mass of subunit B increases in step with the transition to a monomer-like conformation and the loss of intersubunit contacts. (B) Ribbon diagrams of the terminal subunit B at 40-ns intervals showing movements around the tether formed by the D-loop of subunit B stably bound to subunit B-2. Data are from simulation 1. (C) Boxplots of the center of mass RMSF for each subunit in the 13-mer reveal gradients of fluctuations from both ends of the filament. Barbed end subunits with bound ATP fluctuate the most and those with ADP fluctuate the least. Data come from times greater than 200 ns in simulations 1 to 3 for ATP, simulations 4 to 6 for ADP-Pi, and simulations 7 to 9 for ADP (SI Appendix, Table S1).