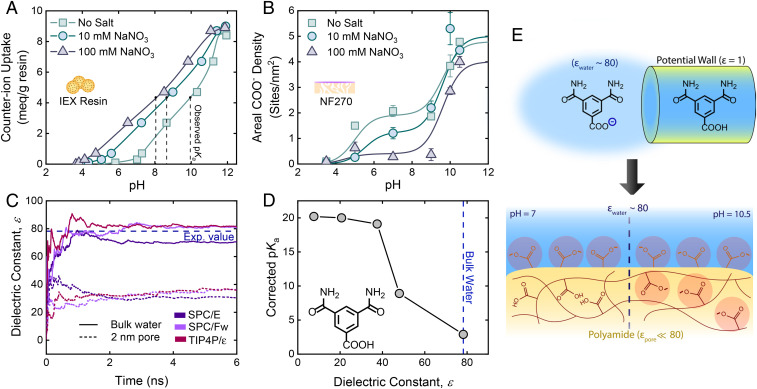
Fig. 3.
Elucidating the mechanisms behind R-COOH ionization. (A) Shift in observed pKa of a carboxylate-based IEX resin due to ionic strength. (B) Effect of ionic strength on the charge state of R-COOH in NF270. Leftward shifts in the observed pKa with respect to ionic strength were not seen. (C) MD simulations of the dielectric constant of bulk and nanoconfined water by the rigid three-site SPC/E, flexible three-site SPC/Fw, and rigid four-site TIP4P/ε water models. (D) Relation between the pKa of a polyamide-based R-COOH analog (3,5-dicarbamoylbenzoic acid) and the dielectric constant of the surrounding media determined by DFT. (E) Relating theoretical insight on dielectric-driven ionization to practical implications. Nanoconfinement of water by a low-permittivity media changes the ionization behavior of R-COOH due to reduced charge stability, measured by enhancements in its pKa (Top). The lower local dielectric constant of confined water found in low-permittivity polyamide pores prevents R-COOH ionization at neutral pH (Bottom Left), whereas at high pH (Bottom Right) the local proton concentration decreases enough to stabilize the excess charge needed for R-COOH ionization. Although depicting a molecularly thin surface, the surface layer likely has some meaningful thickness. All error bars represent one SD.