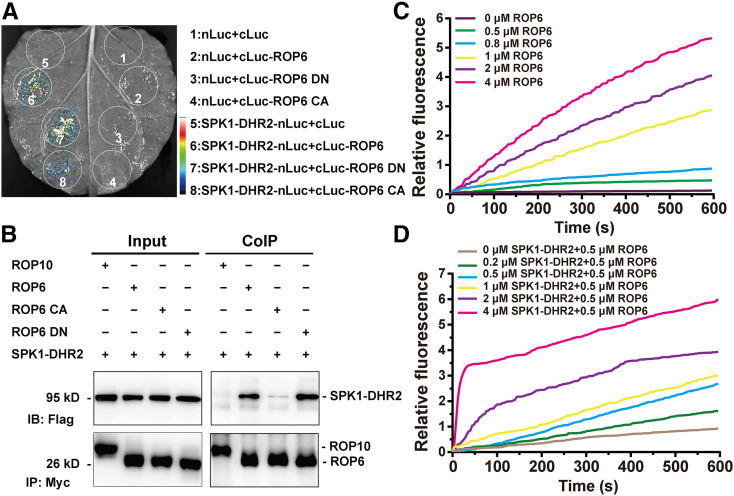
Figure 5.
LjSPK1 Physically Interacts with LjROP6 and Activates Its GTPase Activity In Vitro.
(A) Luciferase biomolecular complementation assays showing the interaction between LjSPK1-DHR2 and LjROP6, LjROP6 CA, or LjROP6 DN in N. benthamiana leaf cells. The indicated constructs were transiently coexpressed in N. benthamiana leaves, and luciferase complementation imaging was captured 2 d after agroinfiltration. nLuc, N-terminal fragment of firefly luciferase; cLuc, C-terminal fragment of firefly luciferase. Fluorescence signal intensity is indicated.
(B) Co-IP assay showing the interaction between LjSPK1-DHR2 and LjROP6 in N. benthamiana leaves. The indicated constructs were coexpressed in N. benthamiana leaves. The co-IP assay was performed using anti-Myc antibody, and the proteins were detected by immunoblot analysis with anti-Flag and anti-Myc antibodies. LjSPK1-DHR2 interacted more strongly with LjROP6 DN than with LjROP6 CA. No interaction was detected between LjSPK1-DHR2 and LjROP10, a type II ROP GTPase.
(C) Time course of intrinsic nucleotide exchange in LjROP6. The intrinsic guanine nucleotide exchange rates of LjROP6 increased with increasing reaction time and increasing concentration of LjROP6. The assays contained 1 μM mant-GTP and the indicated concentration of GDP-LjROP6.
(D) LjSPK1-DHR2 shows concentration-dependent GEF activity toward LjROP6. The assays contained 1 μM mant-GTP, 0.5 μM GDP-LjROP6, and the indicated concentrations of LjSPK1-DHR2.
Results are a representative of three independent assays with similar results.