Figure 6.
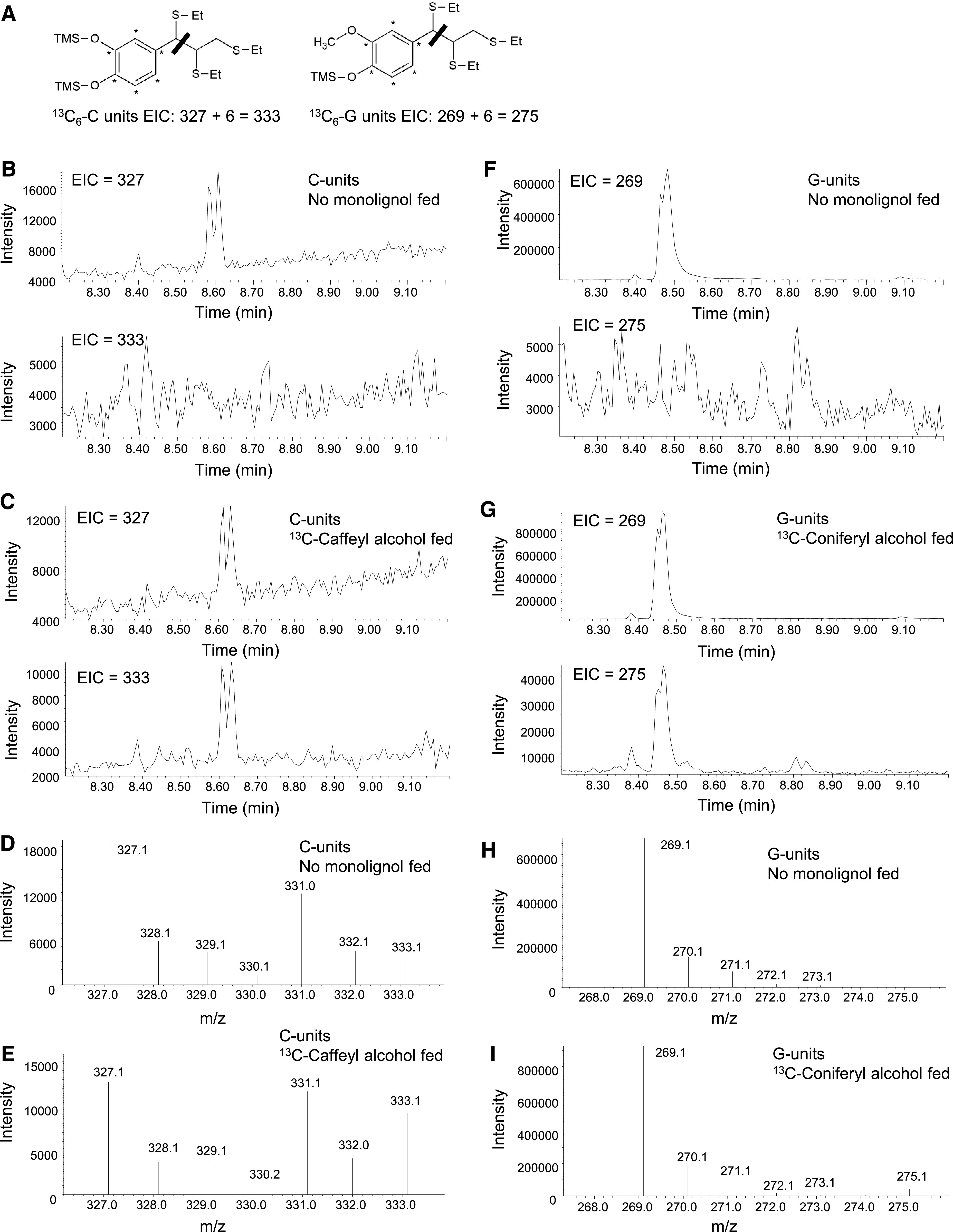
Use of Thioacidolysis to Reveal Lignin Labeling in the 13C Monolignol Feeding Experiments.
(A) Labeling pattern, structures, and masses of monolignol fragments obtained after thioacidolysis from lignin extracted from tissues fed with labeled 13C6-caffeyl alcohol or 13C6-coniferyl alcohol. The thiol group (−SEt) displaces the α-hydroxyl, α-ether, and β-aryl groups during thioacidolysis. The bar indicates the cleavage at the C7-C8 bond during the MS analysis, resulting in the primary fragments at m/z 327 and 269 from the benzylic cation of the trimethylsilylated (TMS) derivatives of unlabeled monolignols and at m/z 333 and 275 of the TMS derivatives of 13C6-labeled monolignols.
(B), (C), (F), and (G) EICs showing 13C-labeled and unlabeled monolignol thioacidolysis products after separation during GC. Double peaks in the EICs represent the presence of threo- and erythro-isomers of the tri-thioetherates shown in (A). EICs of transgenic Medicago hairy root line L6 are deployed here as an example. The no-monolignol–fed sample ([B] and [F]) showed a clear doublet corresponding to the m/z of the unlabeled thioacidolysis products, with no doublet at the m/z of the labeled products. Lignin from samples that were fed with 13C6-caffeyl (C) or 13C6-coniferyl (G) alcohol and could incorporate labeled precursors exhibited both M+6 and unlabeled monolignol-derived products.
(D), (E), (H), and (I) Traces showing the relative abundance of 13C-labeled and unlabeled monolignol thioacidolysis products after fragmentation during MS. Traces correspond to the monolignol thioacidolysis product peaks shown in (B), (C), (F), and (G). Samples that were fed with 13C6-caffeyl (E) or 13C6-coniferyl (I) alcohols showed an increased ratio of M+6 monolignol-derived fragmentation products to the unlabeled ions.
