Abstract
During the COVID-19 pandemic, the effectiveness of the combination of hydroxychloroquine and azithromycin is widely discussed. This treatment can cause many severe cardiac side effects that makes us discuss its utility. The aim of this study is to describe the cardiovascular effect of hydroxychloroquine and azithromycin by analyzing surface ECG in patients with COVID-19. This observational cohort study included Moroccan patients with COVID-19 diagnosis and were hospitalized in Cheikh Khalifa International University Hospital, Casablanca, Morocco between March 26 and April 20, 2020. Patients were treated with a combination of hydroxychloroquine and azithromycin over a period of at least ten days. We were interested in the effects of this combination on the electrocardiogram. A total of 118 eligible patients were enrolled in the study. QT interval prolongation was observed in 19% of patients under the treatment. Only 5 patients required discontinuation of treatment. The factors associated with QT prolongation are male gender (P value 0,043), age over 68 years (P value 0,09), cardiovascular comorbidity (P value 0,013), tisdale score ≥11 (P value < 0,001), and a severe form of COVID-19 (P value < 0,001). First degree atrioventricular block was observed in 2 patients. No serious rhythm or conduction disorders were observed in this study. QT prolongation is a real risk with the combination of hydroxychloroquine and azithromycin. In the current context, it is necessary to select patients at high risk of severe rhythm disturbances that require closer ECG monitoring. Treatment should be discontinued if there are alarming signs such as QTc prolongation beyond 550 ms and the development of ventricular extrasystole or torsade de pointe.
Keywords: Hydroxychloroquine, Azithromycin, COVID-19, Electrocardiogram, QT prolongation
1. Background
The first cases of the new coronavirus disease 2019 (COVID-19) were reported in December 2019 and since the pandemic has spread worldwide [1]. As of May 15, 2020, there were four million confirmed cases and 297,000 deaths worldwide [2].
In China, Chloroquine phosphate is the first drug used against COVID-19 in early clinical studies [3], it was added to the sixth edition of the Guide for the Interim Treatment of COVID-19 [4]. However, based on recent studies, its effectiveness has been claimed [5].
In Morocco, the Ministry of Health, in consultation with the scientific and technical committee of the national program to fight against coronaviruses, decided on 23 March 2020 to adopt the therapeutic protocol based on hydroxychloroquine combined with azithromycin in the various hospitals of the kingdom. And since April 17 as prophylactic treatment in health professionals based on hydroxychloroquine [6].
This combination is currently at the heart of a vast controversy because of its effectiveness still discussed and these potential side effects especially cardiac [7].
Hydroxychloroquine and azithromycin are generally well tolerated medications used in clinical practice, but both can cause QT prolongation [8], their wide use in this epidemic may be hazardous especially in patients with cardiovascular comorbidity.
The aim of this observational study is to describe the electrical effects of the combination of hydroxychloroquine and azithromycin for the treatment of COVID-19 by analyzing the surface ECG and to identify high-risk patients requiring close monitoring or discontinuation of treatment.
2. Methods
2.1. Study design and participants
This observational cohort study included patients residing in Morocco with COVID-19 diagnosis and were hospitalized in Cheikh Khalifa International University Hospital, Casablanca, Morocco between March 26 and April 20, 2020.
Cheick Khalifa International University Hospital, Mohammed VI University of Health Sciences in Casablanca (Morocco) is one of the major authorized hospitals by government for COVID-19 patients. Our study was approved by Ethics Committee of the Mohammed VI University of Health Sciences in Casablanca.
2.2. Diagnosis and grading of COVID-19
All consecutive patients with COVID-19 confirmed by positive Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) results on respiratory specimens admitted to Cheick Khalifa International University Hospital and who were treated with hydroxychloroquine and azithromycin were included. Patients with contraindications to this combination were excluded from this study.
According to the World Health Organization [1], patients can be divided into four types as mild, moderate, severe and critical types. Patients in severe type should meet one of the three criteria: respiratory distress and respiratory rate higher than 30 times per minute; fingertip blood oxygen saturation less than 93% at rest; partial arterial oxygen pressure (PaO2)/fraction of inspiration oxygen (FiO2) less than 300 mmHg. Patients with one of the three conditions are considered as critical type: respiratory failure, requiring mechanical ventilation; shock; multiple organ failure, requiring intensive care management.
In this work, we divided the patients in two groups: non-severe group (mild and moderate types) and severe group (severe and critical types).
2.3. Treatment of COVID-19
From March 26 to April 20, the hospital received 118 patients with confirmed COVID-19 infection. 118 patients received the treatment established by the Ministry of Health: combination of hydroxychloroquine at a dosage of 200 mgX2/day and azithromycin 500 mg on the first day then 250 mgX2 per day for 10 days. All patients had a cardiovascular consultation with ECG prior to treatment and were under clinical, electrical, biological monitoring.
2.4. Data collection
The clinical data of patients were collected from electronic medical records, including demographics, clinical symptoms and signs, co-existing conditions, imaging findings, laboratory results, treatment and clinical outcomes.
A 12-lead ECG is recorded on graph paper, with a standard calibration of the ECG signal, 1 mV = 10 mm and the speed of unwinding of the graph paper of 25 mm/s.
ECG analysis is done manually by two cardiologists who are part of this study (AE, SA). All ECG parameters were analyzed: P-wave measurement, PR interval, QRS, QT interval, T-wave with the appearance of conduction and rhythm disturbance, and repolarization disorder.
QT intervals were measured manually from lead II or V5 or V6 (the longest value being used) of 12-lead ECGs from beginning of the earliest onset of the QRS complex to the end of the T wave. The end of the T wave was determined by extending a tangent from steepest portion of the downslope of the T wave until it crossed the T-P segment. During normal sinus rhythm, QT and RR intervals were averaged over three consecutive complexes. During atrial fibrillation, QT and RR intervals were averaged over all complexes on the 6 s rhythm strips [9–11].
QT intervals were corrected for heart rate using Bazett method and Fridericia: Bazett′s formula is used for heart rates between 60 and 85 bpm, in case of heart rates < 60 bpm or > 85 bpm, Fridericia′s formula is used [10,11].
QTm: measured QT.
The value of normal QTc is variable according to age and gender. A normal QTc interval is < 440 ms in prepuberty, <430 ms in post pubertal males, and <450 ms in post pubertal females [10–12].
In patients with wide QRS(>120 ms), due to underlying intraventricular conduction defects or paced rhythm, we use the following formula to estimate QTc interval: wide QRS adjusted QTc = QTc - (QRS duration - 100 ms) [11,12].
ECGs were recorded in all patients before drug administration and repeated on the second, the fourth, and the sixth day, as well as after treatment. For patients with abnormalities, ECGs were performed at shorter intervals.
We measured the Tisdale score which is a score the risk of QTc prolongation of drug combinations in all patients. This score is based on clinical, biological and electrical data [13] (Table 1). If the score is > 11/21, there is a high risk of drug-associated QT prolongation.
Table 1.
Risk Score For Drug-Associated QTc Prolongation: The Tisdale score [13]. ≤6 predicts low risk, 7–10 medium risk, and ≥11 high risk of drug-associated QT prolongation.
| Risk Factors | Points |
|---|---|
| Age ≥68 y | 1 |
| Female sex | 1 |
| Loop diuretic | 1 |
| Serum K+ ≤3.5 mEq/L | 2 |
| Admission QTc ≥450 ms | 2 |
| Acute MI | 2 |
| If one QTc-prolonging drug | 3 |
| If ≥2 QTc-prolonging drugs | 6 |
| Sepsis | 3 |
| Heart failure | 3 |
| Maximum Risk Score | 21 |
2.5. Statistical analysis
After visually inspecting data, descriptive statistics was carried out. Continuous data were expressed as mean ± standard deviation and categorical parameters were computed as percentages, where appropriate. Based on the normality of data distribution, Student's t-test or the Mann–Whitney U test and chi-squared test were conducted to compare parameters between COVID-19 positive and negative individuals. Figures with p-values equal to or less than 0.05 were considered statistically significant. Statistical analyses were carried out by means of the commercial software “Statistical Package for Social Sciences” (SPSS for Windows, version 24.0, IBM Corp., Armonk, NY, USA).
3. Results
3.1. Demographic and clinical characteristic
Our cohort included 52 female patients (44%), and 66 male patients (55,9%). The average age was 46 years, with 10 patients under 18 years of age, and extremes from 6 years to 86 years.
75 (63%) patients had no particular medical history, 33 (27,9%) patients were hypertensive, 17 (14,4%) patients were diabetic, 8 (6,7%) patients had ischemic heart disease. Three patients had atrial fibrillation (AF): one patient had permanent AF on beta blocker and anticoagulation and two patients had paroxysmal AF on an antiarrhythmic drug (flecainide) and on anticoagulation (Table 2).
Table 2.
Baseline characteristics of the 118 patients.
| VARIABLE | TOTAL |
|---|---|
| Female | 52 (44,06%) |
| Male | 66 (55,93%) |
| Age, mean | 46 years |
| Cardiovascular Risk Factors | |
| Hypertension | 33 (27,96%) |
| Diabetes type I/II | 17 (14,40%) |
| Smoking | 15 (12,71%) |
| Dyslipidemia | 30 (25,42%) |
| Overweight | 12 (10,16%) |
| Known cardiovascular disease | |
| Ischemic heart disease | 8 (6,77%) |
| Coronary bypass surgery | 1 (0,84%) |
| Valvulopathy | 4 (3,38%) |
| Congestive heart failure | 1 (0, 84%) |
| atrial fibrillation | 3 (2,54%) |
| Non-Cardiovascular Medical History | |
| Asthma/Allergy | 8 (6,77%) |
| Hyper or hypothyroidism | 4 (3,38%) |
| Depression/anxiety | 4 (3,38%) |
| Kidney failure | 1 (0,84%) |
| Neoplasm | 2 (1,69%) |
| Vascular cerebral accident | 2 (1,69%) |
| Alcohol | 2 (1,69%) |
| Drug therapy | |
| beta-blockers | 8 (6,77%) |
| ACEI/ARB | 28 (23,72%) |
| calcium Inhibitor | 14 (11,86%) |
| statin | 12 (10,16%) |
| flecainide | 2 (1,69%) |
| amiodarone | 1 (0,84%) |
| Diuretic | 6 (5,06%) |
| Platelet antiaggregant | 15 (12,71%) |
| Antidepressant | 3 (2,54%) |
| Sedative/hypnotics/anxiolytic/anti-psychotics | 2 (1,69%) |
| Salbutamol | 7 (5,93%) |
| COVID-related symptoms | |
| Cough | 39 (33,05%) |
| Fever | 41 (34,74%) |
| Asthenia | 13 (11,01%) |
| Headache | 6 (5,08%) |
| Digestive signs: diarrheal, vomiting | 7 (5,93%) |
| Anosmia | 14 (11,86%) |
| Agnosia | 16 (13,55%) |
| Chest pain | 22 (18,64%) |
| Dyspnea | 22 (18,64%) |
| Palpitation | 11 (9,32%) |
| Unconsciousness | 0 (0%) |
| Asymptomatic | 20 (16,94%) |
| COVID-19 severity | |
| Non-severe group | 79 (66,94%) |
| Patient admitted on reanimation | 39 (33,05%) |
| Death | 9 (7,62%) |
According to the clinical presentation of COVID-19 infection, 79 (66%) patients had a non-severe form and 39 (33%) patients had a severe form, requiring hospitalization in an intensive care unit. 55 (46%) patients were completely asymptomatic, and 24 (20%) patients complained of moderate symptoms like agnosia and anosmia. 41 (34%) patients had fever and 39 (33%) patients had a dry cough. No direct cardiac damage related to COVID-19 was reported in our study.
3.2. Electrocardiogram
We analyzed and compared the ECG performed before and after the initiation of treatment (after 2, 4 and 6 days).
The results are presented in the Table 3.
Table 3.
ECG parameter variability before and after treatment.
| ECG before treatmenta | ECG after treatmenta | p-valueb | |
|---|---|---|---|
| P wave (ms) | 82,25 ± 15,7 | 84,59 ± 15,2 | 0,091 |
| PR (ms) | 150,11 ± 26,3 | 153,64 ± 28,9 | 0,036 |
| QRS duration (ms) | 85,72 ± 17,2 | 88,53 ± 18,4 | 0,013 |
| Corrected QT (ms) | 427,33 ± 39,2 | 443,70 ± 44,4 | <10−4 |
| T wave (ms) | 427,33 ± 39,2 | 443,70 ± 44,4 | <10−4 |
Data indicated as mean ± SD.
Paired samples t-tests for within-group comparisons of ECG changes before and after treatment.
a. The PR interval
Two patients presented with an extension of the PR interval. No atrioventricular conduction disorder was found.
b. The QT interval
The prolonged corrected QT is defined as >460 ms in prepuberty, > 450 ms in post pubertal males and >470 ms in post pubertal females [12].
After hydroxychloroquine and azithromycin combination treatment, 23 (19,49%) patients experienced QT prolongation. 18 patients (15.2%) increased their QTc by more than 40 ms (Fig. 1). QT prolongation occurs more in men, and in patients over 68 years of age. Hypokalemia was presented in only one patient with QTc prolongation.
Fig. 1.
Individual changes in corrected QT (QTc) interval.
In patients with prolonged QTc, Cardiovascular comorbidity (hypertension, diabetic, heart diseases) was present in 34%, the Tisdale score was ≥ to 7 in all patients. It was ≥11 in 5 patients.
69.5% of patients with QT prolongation were in severe COVID-19 infection, 4 of whom died as a result of the infection. The characteristics of patients with QTc prolongation are presented in Table 4.
Table 4.
Characteristics of patients with QTc prolongation.
| QTc normal (n = 95) | Prolonged QTc (n = 23) | P value | |
|---|---|---|---|
| Gender: | 0,043 | ||
| Female | 47 (49,47%) | 6 (26,08%) | |
| Male | 48 (50,52%) | 17 (73,91%) | |
| Age> 68 | 11 (11,57%) | 7 (30,43%) | 0,014 |
| kalemia | 0,82 | ||
| hypokalemia | 2 (2,10%) | 1 (4,34%) | |
| hyperkalemia | 3 (3,15%) | 1 (4,34%) | |
| Cardiovascular comorbidity | 7 (7,36%) | 8 (34,78%) | 0,013 |
| Tisdale risk score | <0,001 | ||
| ≥6 | 77 (81,05%) | 9 (39,13%) | |
| Between 7 and 10 | 16 (16,84%) | 9 (39,13%) | |
| ≥11 | 2 (2,10%) | 5 (21,73%) | |
| Initial QTc ≥500 ms | 1 (1,05%) | 5 (21,73%) | <0,001 |
| Severe form of COVID-19 | 17 (17,89%) | 16 (69,56%) | <0,001 |
The mean time to onset of these electrical signs was 2 days after the start of treatment.
Treatment was discontinued in 5 patients who had a prolonged QTc beyond 550 ms, with the appearance of ventricular extrasystole (VES) in two of them. No torsades de pointes (TdP) or severe rhythm disorders occurred.
c. Rhythm disorder
Four patients had ventricular extrasystole: Two patients had left delayed monomorphic VES without QTc prolongation, both patients are being followed for ischemic heart disease under treatment.
The other two patients had isolated monomorphic VES without bigeminism or trigeminism, their QTc increased from 525 ms to 546 ms for the first patient, and from 508 ms to 560 ms for the second patient, there was no dyskalemia. The combination of hydroxychloroquine and azithromycin was stopped.
A patient who had a paroxysmal AF under flecainide and anticoagulant was admitted initially in sinus rhythm. He had a long QT (540 ms) on the second day of with recurrence AF despite being on flecainide. The combination of hydroxychloroquine and azithromycin was stopped. The patient returned to a sinus rhythm and to a normal QT space.
A patient with permanent AF on betablocker and anticoagulant therapy had a reduction in AF and returned to sinus rhythm without QT prolongation.
d. Repolarization disorder
T-wave elongation was observed in 30%. U wave exaggeration was observed in 19% of patients.
e. Morphological abnormality
P-wave elongation was observed in 5%.
No electrical signs of left ventricular hypertrophy were observed after treatment. (Cornell and Sokolow indexes increased by 1%)
The heart axis hasn't changed.
4. Discussion
Hydroxychloroquine have a long-standing history in the prevention and treatment of malaria and the treatment of chronic inflammatory diseases including systemic lupus erythematosus and rheumatoid arthritis. And for its antiviral action, it is used in the treatment of COVID-19 [14,15].
The toxic effects of hydroxychloroquine are well detailed and described in the literature. Cardiac toxicity of hydroxychloroquine is uncommon, it can be either a rhythm disorder with prolonged QTc and risk of sudden death, or conduction disorder, or cardiomyopathy. These toxic effects depend on the duration of exposure and cumulative dose, so there may be acute toxicity from high doses or toxicity from chronic exposure [16].
In the context of COVID-19, the wide prescription of hydroxychloroquine is not safe: hydroxychloroquine is prescribed at high doses for a shortened duration in combination with azithromycin, also known for its proarrhythmic properties, and this in patients who may have comorbidities, or a severe infectious form [17].
QTc prolongation du to hydroxychloroquine are the consequence of the quinidine-like membrane stabilizing action, type IA according to the Vaughan and Williams classification. It is mainly due to the blocking of the sodium channels of the cardiac cell [18].
QT prolongation was observed in 19% of patients, which is slightly higher to what has been reported in patients with COVID-19 receiving hydroxychloroquine in a U.S. study (11.0%) or a Brazilian study (15%) [19,20].
QTc prolongation is dose-dependent, Borba et al. conducted clinical trial testing two doses of chloroquine in patients with COVID-19. They planned to include 440 patients but stopped after 81 patients were enrolled due to excessive QTc prolongation and a higher mortality in the high-dose group (patients receiving 1200 mg daily for 10 days) compared to the low-dose group (in which patients received 450 mg daily for 4 days after an initial dose of 900 mg on the first day) [20]. QTc prolongation was observed in 11% of patients on low dose, and in 18.9% of patients on high dose. However, in this study they used chloroquine diphosphate which is more toxic than hydroxychloroquine, and patients received in addition to chloroquine diphosphate, azithromycin, ceftriaxone, and Oseltamivir who also have been implicated in QTc prolongation and proarrhythmic events. The COVID-19 forms were more severe.
In our study, severe and critical forms of COVID-19 are less frequent, with a slightly lower mortality rate.
The factors associated with QTc prolongation were: age >68 years, male gender, cardiovascular comorbidity, Tisdale score ≥11, and a severe form of COVID-19.
QTc prolongation is more frequent in men (odds ratio 2.8; P value 0,043), it is similar to what has been described in Chorin study [19]. In the clinical trial by Borda et al. [20], frequency was similar between men and women. Apart from COVID-19, Tisdale reported a more frequent risk of QTc prolongation in women [13].
Age over 68 years is a factor associated with QTc prolongation as reported in several studies (P value 0,014). Cardiotoxicity may be enhanced by older age, pre-existing cardiac disease and renal insufficiency.
In our series, diabetes alone is not a predisposing factor for QTc prolongation. However, the combination of comorbidities such as hypertension, diabetes, heart diseases and kidney failure are predisposing factors. Conversion enzyme inhibitors and calcium channel blockers have not shown a causal relationship with electrical abnormalities. Dyskalemia is not a predisposing factor for QTc prolongation in our study unlike what was reported by Tisdale et al. [13].
A severe COVID-19 infection form is associated with a high risk of QTc prolongation, which may be explained by sepsis with multivisceral failure. This has been reported in several studies [13,20].
In the context of COVID-19, the addition of azithromycin to hydroxychloroquine is associated with greater QTc prolongation according to recently published studies [21,22].
QTc interval prolongation can be considered as a prognostic criterion for the inherent severity of COVID-19 infection, in addition to the need to discontinue first-line therapy [21].
Other electrical abnormalities described in the literature as severe conduction disorders have not been reported in our series [23]. We observed moderate P-wave elongation and QRS widening, and it is similar to what has been described in the long-term use of the treatment [24].
The Moroccan and American Society of Cardiology recommendation focus on baseline risk assessment, frequent QTc monitoring, and strict cutoffs for therapy cessation [6, 25].
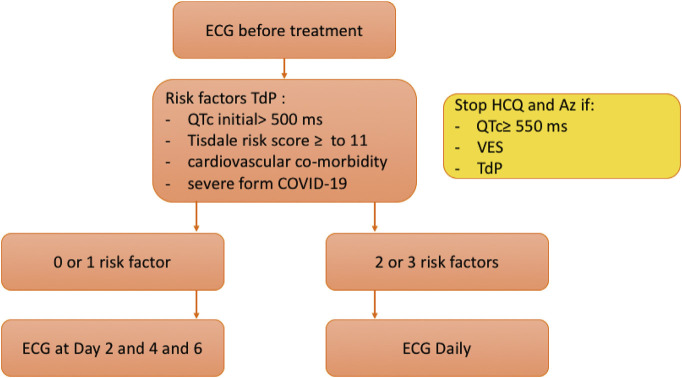
Finally, the introduction of combined hydroxychloroquine and azithromycin therapy requires a rigorous follow-up of the patients, we propose the following protocol for monitoring patients with COVID-19 (Fig. 2).
Fig. 2.
Protocol proposing for electrical monitoring of patients with COVID-19. QTc: corrected QT, VES: ventricular extrasystole, TdP: torsades de pointes.
5. Conclusions
The combination of hydroxychloroquine and azithromycin is not safe in the context of COVID-19. QTc prolongation is a real risk. Therefore, it is necessary to identify high-risk rhythmic patients in whom ECG monitoring should be performed very closely. Treatment should be discontinued if there are any alarming signs: QTc> 550 ms or appearance of VES, or TdP.
6. Limits
The main limitation of the study is the limited number of patients included. Viral cardiac involvement was not completely ruled out in all patients, extensive cardiac workup was performed only in symptomatic patients.
Abbreviations
- ACEI
angiotensin-converting enzyme inhibitors
- AF
atrial fibrillation
- ARB
angiotensin receptor blockers
- AVB
atrioventricular block
- COVID-19
Corona Virus Infectious Disease 2019
- ECG
electrocardiogram
- PCI
percutaneous coronary intervention
- QTm
the measured QT interval
- QTc
the corrected QT interval
- RT-PCR
real-time reverse transcriptase polymerase chain reaction
- TdP
torsades de pointes
- VES
ventricular extrasystole
Author contribution
Conception and design of study: Amal El Ouarradi, Salma Abdeladim, Sara Oualim, Mohamed Sabry. Literature review: Amal El Ouarradi, Salma Abdeladim, Sara Oualim, El Arbi Bouaiti. Acquisition of data: Amal El Ouarradi, Rita Aniq Filali, Ilham Bensahi, Mahassine Elharass, Sara Hafid, El Arbi Bouaiti. Analysis and interpretation of data: Amal El Ouarradi, El Arbi Bouaiti, Abdelhamid Moustaghfir, Mohamed Sabry. Analysis and interpretation of data: Amal El Ouarradi, Abdelhamid Naitlhou, El Arbi Bouaiti. Research investigation and analysis: Amal El Ouarradi, Rita Aniq Filali, Ilham Bensahi, Mahassine Elharass, Hamza Tazi. Research investigation and analysis: Amal El Ouarradi, El Arbi Bouaiti, Abdelhamid Moustaghfir. Drafting of manuscript: Amal El Ouarradi, Salma Abdeladim, Rita Aniq Filali, Ilham Bensahi, Mahassine Elharass. Revising and editing the manuscript critically for important intellectual contents: Amal El Ouarradi, Salma Abdeladim, Sara Oualim, Sara Hafid, Hamza Tazi, Abdelhamid Naitlhou. Data preparation and presentation: Amal El Ouarradi, Sara Hafid, Hamza Tazi, El Arbi Bouaiti. Supervision of the research: Abdelhamid Naitlhou, El Arbi Bouaiti, Mohamed Sabry. Research coordination and management: Abdelhamid Moustaghfir.
Declaration of interests
All authors declare no competing interests.
References
- 1.World Health Organization WHO Director-General's opening remarks at the media briefing on COVID-19. 2020 Mar 11; Available online at: https://www.who.int/dg/speeches/detail/whodirector-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
- 2.COVID-19map. https://coronavirus.jhu.edu/map.html. [Accessed 15 May 2020]
- 3.Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. BioSci Tren. 2020 doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 4.National Health Commission Interpretation of the sixth edition of the guidance for COVID-19 prevention, control, diagnosis, and management [in Chinese] http://www.nhc.gov.cn/xcs/fkdt/202002/54e1ad5c2aac45c19eb541799bf637e9.shtml. [Accessed 2 April 2020]
- 5.Roustit M, Guilhaumou R, Molimard M, Drici MD, Laporte S, Montastruc JL, et al. Chloroquine and hydroxychloroquine in the management of COVID-19: much kerfuffle but little evidence. Therapie. 2020(20):S0040–5957. 30100–1. doi: 10.1016/j.therap.2020.05.010. [published online ahead of print, 2020 May 23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. http://www.covidmaroc.ma/Pages/AccueilAR.aspx.
- 7.Guastalegname M, Vallone A. Could chloroquine/hydroxy-chloroquine be harmful in Coronavirus Disease 2019 (COVID-19) treatment? Clin Infect Dis. 2020 Mar 24; doi: 10.1093/cid/ciaa321. [DOI] [PMC free article] [PubMed]
- 8. www.crediblemeds.org.
- 9.Saqué V, Vaglio M, Funck-Brentano C, Kilani M, Bourron O, Hartemann A, et al. Fast, accurate and easy-to-teach QT interval assessment: the triplicate concatenation method. Arch Cardiovasc Dis. 2017;110(8–9):475–81. doi: 10.1016/j.acvd.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Vandenberk B, Vandael E, Robyns T, Vandenberghe J, Garweg C, Foulon V, et al. Which QT correction formulae to use for QT monitoring? J Am Heart Asso. 2016;5(6):e003264. doi: 10.1161/jaha.116.003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldenberg I, Moss Aj, Zareba W. QT interval: how to measure it and what is “normal. J Cardiovasc Electrophysiol. 2006;17(3):333–6. doi: 10.1111/j.1540-8167.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 12.Rautaharju PM, Surawicz B, Gettes LS. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. J Am Coll Cardiol. 2009;53(11):982–91. doi: 10.1016/j.jacc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Tisdale JE, Jaynes HA, Kingery JR, Mourad NA, Trujillo TN, Overholser BR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circulation: Cardiovas Qual Outc. 2013;6(4):479–87. doi: 10.1161/circoutcomes.113.000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrezenmeier E, Dorner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16(3):155–66. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 15.Zhaowei C, Jijia H, Zongwei Z, Shan J, Shoumeng H. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv. 2020 doi: 10.1101/2020.03.22.20040758. [DOI] [Google Scholar]
- 16.Chatre C, Roubille F, Vernhet H, Jorgensen C, Pers YM. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf. 2018 doi: 10.1007/s40264-018-0689-4. [DOI] [PubMed]
- 17.Kapoor A, Pandurangi U, Arora V, Gupta A, Jaswal A, Nabar A, et al. Cardiovascular risks of hydroxychloroquine in treatment and prophylaxis of COVID-19 patients: a scientific statement from the Indian Heart Rhythm Society. Indian Pacing Electrophysiol J. 2020 doi: 10.1016/j.ipej.2020.04.003. [DOI] [PMC free article] [PubMed]
- 18.Haeusler IL, Chan XHS, Guérin PJ, White NJ. The arrhythmogenic cardiotoxicity of the quinoline and structurally related antimalarial drugs: a systematic review. BMC Med. 2018;16(1) doi: 10.1186/s12916-018-1188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chorin E, Dai M, Shulman E. The QT interval in patients with SARS-CoV-2 infection treated with hydroxy-chloroquine/azithromycin. MedRxiv. 2020 doi: 10.1101/2020.04.02.20047050. . Published April 3 2020[Accessed 20 April 2020] [DOI] [Google Scholar]
- 20.Borba MGS, Val FFA, Sampaio VS, Alexandre MA, Melo G, Brito G, et al. Effect of high vs low doses of chloro- quine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3(4):e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 21.Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed]
- 22.Bessière F, Roccia H, Delinière A, Charrière R, Chevalier P, Argaud L, et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. 2020:e201787. doi: 10.1001/jamacardio.2020.1787. [published online ahead of print, 2020 may 1] [DOI] [PMC free article] [PubMed]
- 23.White NJ. Cardiotoxicity of antimalarial drugs. Lancet Infect Dis. 2007;7(8):549–58. doi: 10.1016/s1473-3099(07)70187-1. [DOI] [PubMed] [Google Scholar]
- 24.McGhie TK, Harvey Su PJ, Anderson N, Tomlinson G, Touma Z. Electrocardiogram abnormalities related to anti-malarials in systemic lupus erythematosus. Clin Exp Rheumatol. 2018;36:545–51. [PubMed] [Google Scholar]
- 25.Giudicessi JR, Noseworthy PA, Friedman PA, Ackerman MJ. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19) Mayo Clin Proc. 2020 doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed]