Abstract
Background
This study is to describe the changes in prescribing practices of antibiotics to treat community-acquired pneumonia (CAP) in Korea during 2010–2015.
Methods
The claim database of the Health Insurance Review and Assessment Service in Korea was used to select adult patients (≥ 18 years of age) admitted between 2010 and 2015, with the International Classification of Diseases, Tenth Revision codes relevant to all-cause pneumonia for the first or second priority discharge diagnosis. The episodes with hospital-acquired or healthcare-associated pneumonia were excluded. Consumption of each antibiotic was converted to defined daily dose (DDD) per episode. The amount of antibiotic consumption was compared between patients with CAP aged < 65 years and those aged ≥ 65 years.
Results
The average amount of antibiotic consumption per episode was 15.5 DDD, which remained stable throughout the study period (P = 0.635). Patients aged ≥ 65 years received more antibiotics than those aged < 65 years (15.7 vs. 15.3 DDD). Third-generation cephalosporin (4.9 DDD/episode, 31.4%) was the most commonly prescribed, followed by macrolide (2.7 DDD/episode, 17.1%) and beta-lactam/beta-lactamase inhibitor (BL/BLI) (2.1 DDD/episode, 13.6%). The consumption amount of fourth-generation cephalosporin (4th CEP) (P = 0.001), BL/BLI (P = 0.003) and carbapenem (P = 0.002) increased each year during the study period. The consumption of 4th CEP and carbapenem was doubled during 2010–2015.
Conclusion
The prescription of broad-spectrum antibiotics such as 4th CEP and carbapenem to treat CAP increased in Korea during 2010–2015.
Keywords: Antibiotics, Pneumonia, Resistance, Stewardship, Korea
Graphical Abstract
INTRODUCTION
Antimicrobial-resistant pathogens erode the effectiveness of antibiotics, leading to higher mortalities, longer hospital stays, and increased medical costs.1 Approximately 2.8 million individuals develop an infection and more than 35,000 people die annually in the U.S. due to widespread antimicrobial-resistant pathogens.2 The emergence of antimicrobial-resistant organisms is considered to be a serious threat to public health, and the importance of proper antibiotic usage has been emphasized to cope with the threat.3
Unfortunately, Korea is known to have a high level of antibiotic prescription among the Organization for Economic Cooperation and Development countries.4 The use of antibiotics, especially broad-spectrum antibiotics, has been increasing in the last decade in Korean hospitals.5,6 Accordingly, the National Action Plan on Antimicrobial Resistance in Korea set as their primary objective a prudent use of antibiotics and reduction of unnecessary antibiotics prescriptions.7 The first step toward implementing a proper policy for antimicrobial stewardship is identifying the current situation. Although some quantitative studies have already been conducted, more extensive and far-reaching analyses on antibiotic usage patterns are needed.
Community-acquired pneumonia (CAP) is one of the most common community-acquired bacterial infections, which usually requires an antibiotic treatment.8 In Korea, the most causative bacterial pathogen for CAP is Streptococcus pneumoniae, which comprises 27%–69% of the total isolated bacteria.8 Fortunately, the resistance rate of S. pneumoniae to ceftriaxone and fluoroquinolone (FQ) is less than 10%.9,10 However, some reports demonstrated that the proportion of cases due to gram-negative bacteria and S. aureus was higher in elderly patients, which raised concern about antimicrobial resistance.11
Although efforts to improve microbiological diagnosis for patients with CAP are still ongoing, the rate of microbiological diagnosis remains around 40% due to difficulties in securing adequate samples.12,13 Hence, selecting antibiotics to treat CAP is highly dependent on the official guidelines based on empirical antibiotic treatments. The treatment guidelines for CAP are developed based on local epidemiology, but individual physicians may not adhere to them due to the governmental policies regarding restrictions on antimicrobial agents, prescription behavior of each physician, etc.
Analyzing the antibiotic prescription patterns of CAP may give a clue to the changing patterns in the practices of prescribing antibiotics to treat common bacterial infections in Korea, which is the goal of this study as viewed more broadly.
METHODS
Data source
The National Health Insurance System of Korea covers almost the entire population, including low-income families receiving medical aids; approximately 98% of the population is covered by the system.14 We obtained the National Health Insurance claim data through the Healthcare Big Data Hub, where the Health Insurance Review & Assessment Service provides the health insurance data online.
The data include age, sex, insurer type, clinic/hospital code, area code of clinic/hospital, care type (inpatient/outpatient), treatment start date, treatment duration, admission or visit days, primary discharge diagnosis code, sub-discharge diagnosis code, medical department in charge, medical cost, and prescribed pharmaceuticals. The discharge diagnoses were coded following the International Classification of Diseases, Tenth Revision (ICD-10). To identify the underlying comorbidities of patients and to minimize missing variables due to the lag in billing for health insurance claims, the claim data were obtained from January 2009 to December 2016.
Definitions
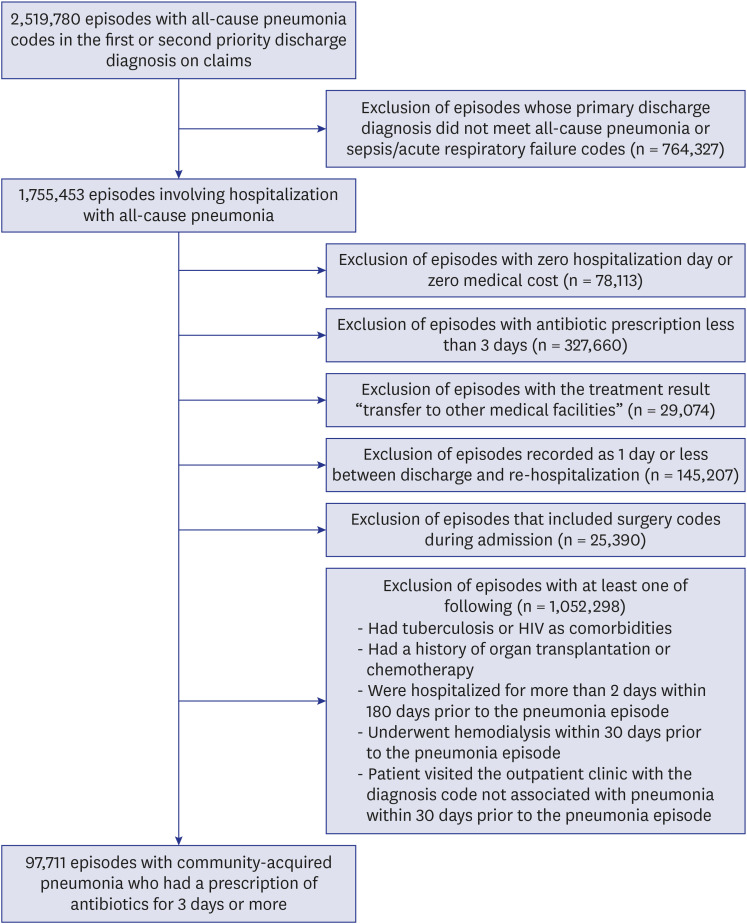
We examined the episodes for adult (≥ 18 years) hospitalized patients with ICD-10 codes for all-cause pneumonia in the first or second priority discharge diagnosis.6,15 All-cause pneumonia codes used in the present study are presented in Supplementary Table 1. We included the codes relevant to viral pneumonia (J10, J11, and J12), because empirical antibiotics are likely to be applied to any type of pneumonia before causative pathogens are revealed. The episodes with zero hospitalization day or zero medical cost, as well as those with antibiotic prescription for less than 3 days were excluded.16 In addition, the episodes with the treatment result “transfer to other medical facilities” and those with 1 day or less between discharge and re-hospitalization were excluded.
Importantly, episodes that could be classified as hospital-acquired or healthcare-associated pneumonia were excluded, which had any of the following characteristics. Surgery codes were recorded during admission; patients had tuberculosis or acquired immune deficiency syndrome (AIDS)/human immunodeficiency virus (HIV) as comorbidities; patients had a history of organ transplantation or chemotherapy; patients were hospitalized for more than 2 days within 180 days prior to the pneumonia episode; patients underwent hemodialysis within 30 days prior to the pneumonia episode; and patients visited the outpatient clinic with the diagnosis code not associated with pneumonia within 30 days prior to the pneumonia episode (Fig. 1).17,18,19
Fig. 1. Flow diagram showing the process of selecting episodes with community-acquired pneumonia based on the National Health Insurance claims data.
HIV = human immunodeficiency virus.
The comorbidities examined in this study were based on the disease categories in the Charlson's comorbidity index, and they were myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, rheumatic disease, moderate or severe liver disease, hemiplegia or paraplegia, renal disease, any malignancy (except malignant neoplasm of the skin), metastatic solid tumor, AIDS/HIV, and dementia.20
“Medical costs” included all expenses paid by the patient or the health insurance system, such as cost per prescription, hospitalization charge, procedure charge, laboratory test charge, and radiologic examination charge. Non-reimbursed medical costs were not included. All costs are in the US dollars (1 USD ≒ 1,150 KRW).
Antibiotics were defined as medication with Anatomical Therapeutic Chemical (ATC) class J01, which does not include antifungal agents or antituberculosis agents. Systemic agents with per oral or parenteral administration route were included, whereas topical agents were excluded. Antibiotics prescribed at hospital discharge were included for analysis. The consumption amount of each class of antibiotics was converted to defined daily dose (DDD) following the ATC classification system of the World Health Organization,21 and then standardized for per episode.
Data analysis and statistics
Incidence rates were calculated using the population size data from the Statistic Korea as the denominator, because the National Health Insurance System of Korea covers almost the entire population. The population size for age ≥ 18 and ≥ 65 in Korea during 2010-2015 was 306,196,939 and 36,734,078, respectively.22 The demographic data, clinical features, and antibiotic usage of the patients with pneumonia were obtained, and these variables were compared between patients with CAP aged < 65 years (the 65- group) and those aged ≥65 years (the 65+ group). For significant differences between groups, we used the t- or χ2 test.
Linear regression models were used to assess the trend of antibiotic consumption over time or age. To allow the trend to vary across different ages, an age quadratic term was included in the regression model. Statistical significance was defined as P < 0.05. All analyses were performed using SAS enterprise guide version 6.1 (SAS Institute Inc., Cary, NC, USA).
Ethics statement
The study protocol was approved by the Institutional Review Board (IRB) of Hanyang University Hospital (IRB No. 2017-01-020), and the requirement for a written informed consent was waived.
RESULTS
Baseline characteristics of patients with CAP
A total of 97,711 CAP episodes were identified in 2010–2015 in Korea (15,418 in 2010, 16,777 in 2011, 17,070 in 2012, 14,882 in 2013, 15,940 in 2014, and 17,624 in 2015). Of them, 58.3% occurred in the 65+ group. The overall incidence per 10,000 persons was 3.19; the incidence rate of the 65+ group was 15.52, which was approximately 10-fold the incidence rate 1.51 of the 65- group.
Table 1 shows the baseline characteristics of patients with CAP. Approximately 56.5% of the total patients were female, and the median age was 73 years with the interquartile range (IQR) 46–83. The most common comorbidities were diabetes mellitus (43.7/100 episodes) and chronic pulmonary disease (43.4/100 episodes), followed by cerebrovascular disease (29.8/100 episodes). The number of comorbidities of the 65+ group is often several times that of the 65- group: 3.9-fold in diabetes mellitus, 1.9-fold in chronic pulmonary disease, and 7.5-fold in cerebrovascular disease. There was a significant difference between the two age groups in all variables used.
Table 1. Baseline characteristics of community-acquired pneumonia patients in Korea during 2010–2015.
| Characteristics | Total | Age, < 65 (41.7%) | Age, ≥ 65 (58.3%) | P valuea | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male (n = 42,456) | Female (n = 55,255) | Total (n = 97,711) | Male (n = 18,733) | Female (n = 21,979) | Total (n = 40,712) | Male (n = 23,723) | Female (n = 33,276) | Total (n = 56,999) | |||
| Age, yr, median (IQR) | 69 (45–81) | 76 (47–85) | 73 (46–83) | 42 (32–54) | 41 (31–53) | 42 (31–53) | 80 (74–85) | 83 (78–88) | 82 (76–87) | ||
| No. of comorbidities,b per 100 CAP episodes | |||||||||||
| Diabetes mellitus | 45.4 | 42.4 | 43.7 | 20.1 | 12.8 | 16.2 | 65.4 | 61.9 | 63.4 | < 0.001 | |
| Chronic pulmonary disease | 43.7 | 43.2 | 43.4 | 26.6 | 29.8 | 28.3 | 57.1 | 52.1 | 54.2 | < 0.001 | |
| Cerebrovascular disease | 30.7 | 29.2 | 29.8 | 8.3 | 4.5 | 6.2 | 48.4 | 45.5 | 46.7 | < 0.001 | |
| Dementia | 22.3 | 27.1 | 25.0 | 2.5 | 1.0 | 1.7 | 37.9 | 44.4 | 41.7 | < 0.001 | |
| Peripheral vascular disease | 18.3 | 19.3 | 18.8 | 5.3 | 5.5 | 5.4 | 28.5 | 28.4 | 28.4 | < 0.001 | |
| Congestive heart failure | 16.4 | 20.9 | 18.9 | 3.9 | 2.4 | 3.1 | 26.3 | 33.1 | 30.3 | < 0.001 | |
| Hemiplegia or paraplegia | 11.6 | 9.6 | 10.5 | 4.4 | 1.3 | 2.8 | 17.3 | 15.1 | 16.0 | < 0.001 | |
| Renal disease | 4.9 | 3.5 | 4.1 | 1.2 | 0.6 | 0.9 | 7.7 | 5.5 | 6.4 | < 0.001 | |
| Moderate or severe liver disease | 2.0 | 1.4 | 1.6 | 1.4 | 0.7 | 1.0 | 2.5 | 1.8 | 2.1 | < 0.001 | |
| Any malignancy | 0.8 | 6.9 | 4.2 | 0.7 | 2.7 | 1.8 | 0.9 | 9.6 | 6.0 | < 0.001 | |
| Metastatic solid tumor | 0.1 | 0.6 | 0.4 | 0.1 | 0.2 | 0.1 | 0.1 | 0.9 | 0.5 | < 0.001 | |
| Average hospitalization, day | 12.3 | 11.7 | 12.0 | 9.6 | 8.8 | 9.2 | 14.4 | 13.6 | 13.9 | < 0.001 | |
| Average antibiotic cost, USD | 228.1 | 187.2 | 204.9 | 156.4 | 124.3 | 139.1 | 284.7 | 228.7 | 252.0 | < 0.001 | |
| Average medical cost, USD | 2,200.9 | 1,781.1 | 1,963.5 | 1,436.6 | 1,061.6 | 1,234.2 | 2,804.5 | 2,256.4 | 2,484.5 | < 0.001 | |
| Proportion of antibiotic cost from total medical cost, % | 10.4 | 10.5 | 10.4 | 10.9 | 11.7 | 11.3 | 10.1 | 10.1 | 10.1 | ||
IQR = interquartile range, CAP = community-acquired pneumonia.
aPatients aged < 65 vs. ≥ 65 years; bSome patients had multiple comorbidities.
The average hospitalization day for CAP episodes was 12.0 days, and the hospitalization duration was longer in the 65+ group than in the 65- group (13.9 vs. 9.2 days, P < 0.001). The average medical cost per hospitalized CAP episode was 1,963.5 USD, and for the 65+ group, it was about twice as high as that for the 65- group (2,484.5 vs. 1,234.2 USD, P < 0.001).
Overall antibiotic consumption in treating CAP
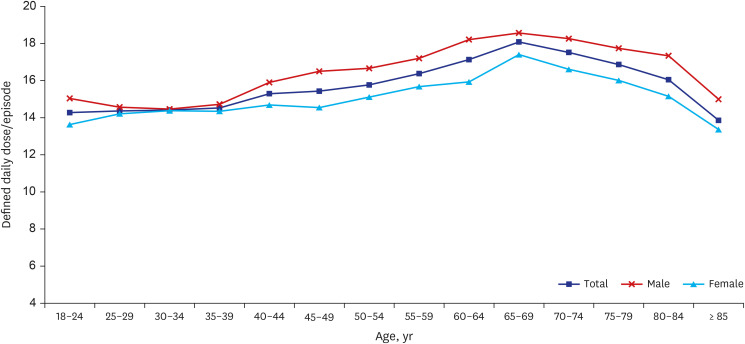
Table 2 shows the overall antibiotic usage pattern for the treatment of CAP in Korea in 2010–2015. The average amount of antibiotic consumption per episode was 15.5 DDD, which remained stable throughout the study period (15.3 DDD in 2010; 15.9 in 2011; 15.3 in 2012; 15.5 in 2013; 15.4 in 2014; and 15.8 in 2015, P = 0.635). Men received more antibiotics than women (16.6 vs. 14.7 DDD, P < 0.001); the 65+ group received more antibiotics than the 65- group (15.7 vs. 15.3 DDD, P < 0.001). The antibiotic use increased up until age 70 (P = 0.005), but then started to decrease beyond age 70 (P = 0.012) (Fig. 2).
Table 2. Overall antibiotic usage pattern for the treatment of community-acquired pneumonia in Korea during 2010–2015.
| Variables | Total | Age, < 65 | Age, ≥ 65 | P valuea | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | Male | Female | Total | |||
| Average antibiotic consumption, DDD/episode | |||||||||||
| Total | 16.6 | 14.7 | 15.5 | 16.0 | 14.7 | 15.3 | 17.1 | 14.8 | 15.7 | < 0.001 | |
| Second-generation cephalosporin | 0.8 | 0.8 | 0.8 | 1.0 | 1.0 | 1.0 | 0.6 | 0.6 | 0.6 | < 0.001 | |
| Third-generation cephalosporin | 4.9 | 4.9 | 4.9 | 4.8 | 4.3 | 4.5 | 5.0 | 5.2 | 5.1 | < 0.001 | |
| Beta-lactam/beta-lactamase inhibitorb | 2.3 | 2.0 | 2.1 | 2.0 | 1.8 | 1.9 | 2.5 | 2.1 | 2.3 | < 0.001 | |
| Fluoroquinolone | 1.6 | 1.3 | 1.4 | 1.7 | 1.4 | 1.6 | 1.4 | 1.2 | 1.3 | < 0.001 | |
| Macrolide | 2.7 | 2.6 | 2.7 | 4.0 | 4.2 | 4.1 | 1.7 | 1.5 | 1.6 | < 0.001 | |
| Carbapenem | 1.6 | 1.0 | 1.3 | 0.6 | 0.3 | 0.4 | 2.3 | 1.5 | 1.8 | < 0.001 | |
| Glycopeptide | 0.7 | 0.4 | 0.5 | 0.3 | 0.1 | 0.2 | 1.0 | 0.6 | 0.8 | < 0.001 | |
| First-generation cephalosporin | 0.6 | 0.8 | 0.7 | 0.8 | 0.9 | 0.9 | 0.5 | 0.7 | 0.6 | < 0.001 | |
| Othersc | 1.4 | 0.9 | 1.1 | 0.8 | 0.7 | 0.7 | 2.1 | 1.4 | 1.6 | < 0.001 | |
| Parenteral administration, % | 60.8 | 60.1 | 60.4 | 47.1 | 44.9 | 45.9 | 70.9 | 70.2 | 70.5 | < 0.001 | |
| Average antibiotic cost, USD | 228.1 | 187.2 | 204.9 | 156.4 | 124.3 | 139.1 | 284.7 | 228.7 | 252.0 | < 0.001 | |
| Proportion of antibiotic cost from total medical cost, % | 10.4 | 10.5 | 10.4 | 10.9 | 11.7 | 11.3 | 10.1 | 10.1 | 10.1 | < 0.001 | |
DDD = defined daily dose.
aPatients aged < 65 vs. ≥ 65 years; bIt includes amoxicillin/clavulanate, amoxicillin/sulbatam, ampicillin/sulbactam, ticarcillin/clavunatae, piperacillin/sulbactam, piperacillin/tazobactam, and sultamicillin; cIt includes fourth-generation cephalosporin, aminoglycoside, nitroimidazole, lincosamide, monobactam, oxazolidinone, penicillin, polymyxin, trimethoprim/sulfamethoxazole, tetracycline, etc.
Fig. 2. Average antibiotic consumption per hospitalized community-acquired pneumonia episode by age group during 2010–2015.
Of the total antibiotic use, 60.4% were administered parenterally. The 65+ group received antibiotics parenterally more than the 65- group did (70.5% vs. 45.9%, P < 0.001). The average antibiotic cost was 204.9 USD, and it decreased every year (235.5 USD in 2010; 216.4 in 2011; 195.9 in 2012; 197.9 in 2013; 191.5 in 2014; and 194.2 in 2015, P = 0.027). The average antibiotic cost for the 65+ group was approximately 1.8-fold that for the 65- group (252.0 vs. 139.1 USD, P < 0.001). The proportion of the antibiotic cost in the total medical cost was approximately 10%, which is similar for the two age groups.
Annual average consumption of antibiotic classes in treating CAP
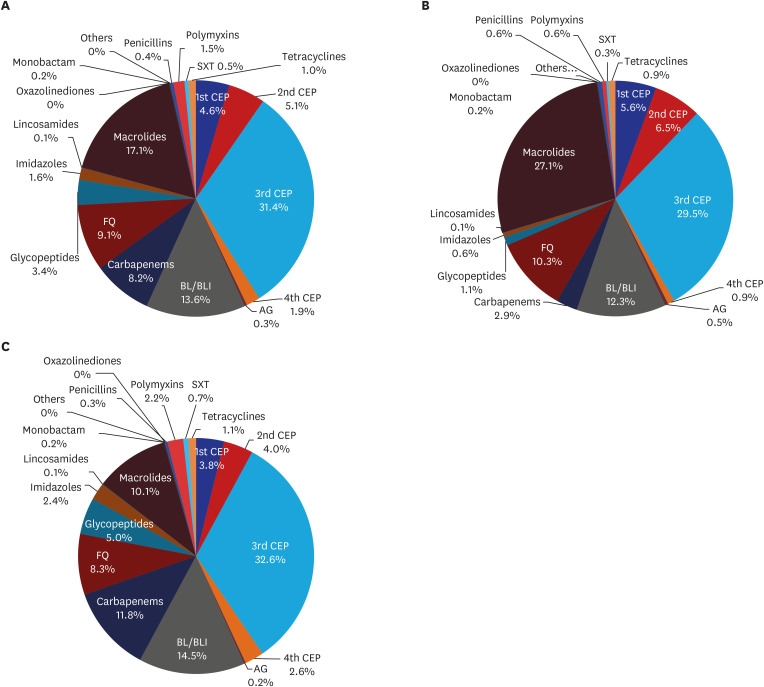
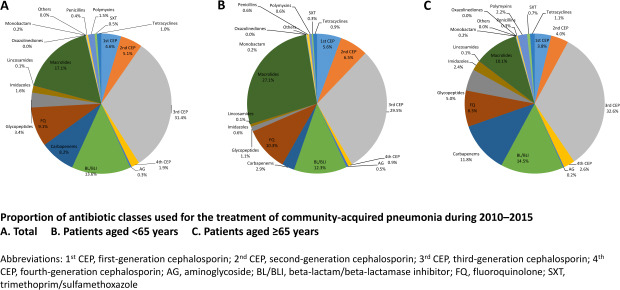
Fig. 3 and Supplementary Table 2 show the proportion of antibiotic classes to treat CAP from 2010 to 2015. Third-generation cephalosporin (3rd CEP) (4.9 DDD/episode, 31.4%) was the most commonly prescribed, followed by macrolide (2.7 DDD/episode, 17.1%) and beta-lactam/beta-lactamase inhibitor (BL/BLI) (2.1 DDD/episode, 13.6%). The three most commonly prescribed antibiotics for the 65- group were 3rd CEP (4.5 DDD/episode, 29.5%), macrolide (4.1 DDD/episode, 27.1%) and BL/BLI (1.9 DDD/episode, 12.3%). Differently from this, 3rd CEP (5.1 DDD/episode, 32.6%), BL/BLI (2.3 DDD/episode, 14.5%) and carbapenem (1.8 DDD/episode, 11.8%) were the most commonly prescribed for the 65+ group.
Fig. 3.
Proportion of antibiotic classes used for the treatment of community-acquired pneumonia during 2010–2015.
(A) Total, (B) Patients aged < 65 years, (C) Patients aged ≥ 65 years.
1st CEP = first-generation cephalosporin, 2nd CEP = second-generation cephalosporin, 3rd CEP = third-generation cephalosporin, 4th CEP = fourth-generation cephalosporin, AG = aminoglycoside, BL/BLI = beta-lactam/beta-lactamase inhibitor, FQ = fluoroquinolone, SXT = trimethoprim/sulfamethoxazole.
Table 3 and Supplementary Fig. 1 present the annual average consumption of broad-spectrum antibiotics to treat CAP. The consumption amount of fourth-generation cephalosporin (4th CEP) (P = 0.001), BL/BLI (P = 0.003) and carbapenem (P = 0.002) increased every year during the study period. As a result, the 4th CEP consumption in 2015 was twice that in 2010; the BL/BLI consumption in 2015 was 1.2-fold that in 2010; and the carbapenem consumption in 2015 was 1.9-fold that in 2010. There was no noticeable change in the usage of 3rd CEP (P = 0.769), FQ (P = 0.299) and glycopeptide (P = 0.159).
Table 3. Annual average consumption of broad-spectrum antibiotics per community-acquired pneumonia episode in Korea during 2010–2015 (unit: defined daily dose/episode).
| Variables | Third-generation cephalosporin | Fourth-generation cephalosporin | Beta-lactam/beta-lactamase inhibitor | Carbapenem | Fluoroquinolone | Glycopeptide | |
|---|---|---|---|---|---|---|---|
| Total | |||||||
| 2010 | 4.79 | 0.19 | 1.96 | 0.85 | 1.41 | 0.47 | |
| 2011 | 5.00 | 0.25 | 1.90 | 0.91 | 1.43 | 0.45 | |
| 2012 | 4.75 | 0.30 | 2.05 | 1.15 | 1.33 | 0.52 | |
| 2013 | 4.91 | 0.29 | 2.20 | 1.53 | 1.41 | 0.64 | |
| 2014 | 4.90 | 0.35 | 2.23 | 1.52 | 1.42 | 0.58 | |
| 2015 | 4.87 | 0.38 | 2.34 | 1.64 | 1.51 | 0.54 | |
| P value | 0.769 | 0.001 | 0.003 | 0.002 | 0.299 | 0.159 | |
| Age, < 65 yr | |||||||
| 2010 | 3.99 | 0.07 | 2.01 | 0.36 | 1.56 | 0.15 | |
| 2011 | 4.55 | 0.11 | 1.86 | 0.33 | 1.59 | 0.16 | |
| 2012 | 4.06 | 0.12 | 1.91 | 0.40 | 1.40 | 0.16 | |
| 2013 | 4.58 | 0.16 | 1.94 | 0.60 | 1.53 | 0.23 | |
| 2014 | 4.84 | 0.18 | 1.78 | 0.46 | 1.60 | 0.18 | |
| 2015 | 5.07 | 0.21 | 1.79 | 0.56 | 1.71 | 0.19 | |
| P value | 0.030 | < 0.001 | 0.065 | 0.069 | 0.335 | 0.205 | |
| Age, ≥ 65 yr | |||||||
| 2010 | 5.37 | 0.28 | 1.93 | 1.20 | 1.30 | 0.69 | |
| 2011 | 5.43 | 0.39 | 1.93 | 1.46 | 1.27 | 0.73 | |
| 2012 | 5.24 | 0.42 | 2.15 | 1.68 | 1.28 | 0.77 | |
| 2013 | 5.11 | 0.37 | 2.36 | 2.07 | 1.33 | 0.88 | |
| 2014 | 4.94 | 0.47 | 2.52 | 2.21 | 1.29 | 0.85 | |
| 2015 | 4.73 | 0.50 | 2.73 | 2.40 | 1.37 | 0.79 | |
| P value | 0.002 | 0.020 | < 0.001 | < 0.001 | 0.153 | 0.103 | |
The consumption of 3rd CEP (P = 0.030) and 4th CEP (P < 0.001) increased for the 65- group. For the 65+ group, however, the consumption of 3rd CEP (P = 0.002) decreased, whereas the consumption of 4th CEP (P = 0.020), BL/BLI (P < 0.001) and carbapenem (P < 0.001) increased.
DISCUSSION
The type of antibiotics used to treat CAP mostly depends on empirical therapies due to difficulties in isolating the causative pathogens.8 Accordingly, guidelines for empirical therapies influence the selection of antibiotics for CAP. The 2009 Korean guideline for CAP treatment recommended that the first-line empirical antibiotic for patients hospitalized in general wards should be FQ alone or beta-lactam combined with macrolide.23 These regimens were effective for Streptococcus pneumoniae and Myoplasma pneumoniae which were the most common causative agents of CAP in Korea, and the recommendation was in place for many years.13 However, macrolide was no longer recommended in the 2018 revised guideline, because no significant difference was seen in the treatment outcomes between beta-lactam alone and beta-lactam combined with macrolide.8 The guidelines corroborate this study's finding that the most commonly prescribed antibiotic classes are 3rd CEP, macrolide, and BL/BLI.
The possible reason for the preference of beta-lactam and macrolide to FQ is as follows. First, FQ for patients with CAP might delay the diagnosis of tuberculosis and increase the risk of antibiotic resistance. Hence, physicians are cautious in using FQ to treat CAP in Korea where the prevalence of tuberculosis is not low.24,25 Second, safety concerns about FQ might have discouraged physicians from using FQ as frequently as other antibiotics. In fact, the US Food and Drug Administration has been warning about FQ usage since 2008 due to the risk of tendinitis, aortic rupture, QT prolongation, etc.26
Older adult patients received a higher amount of antibiotics and more broad-spectrum antibiotics than younger adult patients. Although the recommendation for empirical antibiotics for CAP in older patients follows the standard guideline, the risk of antimicrobial-resistant pathogens weighs more heavily for older patients due to the higher numbers of comorbidities and frequent histories of hospitalization.8,27 In addition, the causative organisms of CAP in older patients tend to be polymicrobial with gram-negative bacteria, particularly in those who have a chronic pulmonary disease. These possibly lead physicians to prescribing a higher amount of broad-spectrum antibiotics for older patients.28 Another possible explanation for the discrepancy between older and younger patients is the incidence of aspiration pneumonia. Aspiration is an important mechanism of CAP in older adults, especially in those with cognitive or functional impairment.29 Accordingly, Korean physicians tend to use a combination of antibiotics for anaerobes more frequently in older patients than in younger patients.30
Also noticeable in this study is the change in the antibiotic prescription pattern: the consumption amount of 4th CEP and carbapenem almost doubled in 2015, compared with that in 2010. Given that the local guideline for CAP empirical therapy did not change during the study period, the result of this study seems to reflect the change in prescribing practices in the medical community in Korea. The increase in the number of MDR gram-negative pathogens that are frequently found in hospital-acquired pneumonia (HAP) could be a possible explanation for this phenomenon.31 In fact, the rate of antibiotic resistance of S. pneumoniae isolated from any kind of specimen in Korea did not change considerably during the study period,31,32 while the resistance of gram-negative pathogens such as Klebsiella pneumoniae or Escherichia coli to 3rd CEP or FQ increased significantly.33,34 Physicians frequently encountered MDR gram-negative pathogens from HAP, and characteristics of infections in elderly patients with comorbidities might be similar to hospital-acquired infections because of frequent hospitalization.35 Indeed, prescription of carbapenem and 4th CEP increased in the patients over 65 years in this report. To clarify this issue, it is necessary to study the causative pathogens and their antimicrobial susceptibility in CAP elderly population in Korea.
The main strength of the present study is the data source. Using the National Health Insurance Data, which is the most representative and extensive health data in Korea covering almost all citizens, we showed clearly the overall changes in the practices of prescribing antibiotics as a treatment for common bacterial infections. However, this study has some limitations. First, our data do not contain laboratory or radiologic results. Using the administrative data alone, we could not validate the diagnosis, and the correlation between the changes in antimicrobial-resistant pathogens and the antibiotic prescription patterns could not be evaluated. Second, our exclusion criteria were so strict that the estimated incidence of CAP was much lower than that reported in previous studies. A Korean study using the same data source as used in the present study reported that the incidence of adult (≥ 19 years) hospitalized CAP was estimated to be 62.6 per 10,000 persons.36 Our exclusion criteria were set as strictly as possible to select the genuine CAP episodes while minimizing the possibility of including hospital-acquired or healthcare-associated pneumonia. Although several CAP cases might have been excluded inadvertently, we believe that our data provided a good approximation to the true antibiotic consumption value for CAP in Korea. Third, our measurement unit ‘DDD/episode’ might have been influenced by the severity of the disease. Early death in older age groups may have resulted in a lower antibiotic consumption. Fourth, sub-analyses according to the disease severity were lacking. Because antibiotic consumption might be influenced by the disease severity, it would have been better to analyze according to the admission location such as the general ward or intensive care unit. Finally, the antibiotic prescription was evaluated only with DDD. According to a recent guideline, “days of therapy” is preferred to DDD as a measure of antibiotic consumption.37 Due to the nature of National Health Insurance Data which include only the essential information for claims, however, detailed information on antibiotic prescription such as duration or combined antibiotic therapy was not available.
In conclusion, the prescription of broad-spectrum antibiotics such as 4th CEP and carbapenem increased for the treatment of CAP in Korea during 2010–2015, which was particularly noticeable for older patients than for young patients.
Footnotes
Funding: This work was supported by a grant from the Korea Healthcare Technology R&D Project, Nationwide surveillance system of multidrug-resistant pathogens for prevention and control of antimicrobial resistance in Korea (HI12C0756), Ministry of Health and Welfare, Republic of Korea; BCWorld Pharm. Co. Ltd. (2019) also partly funded this research.
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Pai H.
- Data curation: Kim B, Myung R.
- Formal analysis: Myung R.
- Funding acquisition: Pai H.
- Investigation: Kim B, Myung R.
- Methodology: Kim B, Lee MJ, Pai H.
- Project administration: Kim B, Pai H.
- Resources: Kim B, Myung R, Lee MJ, Kim J, Pai H.
- Software: Myung R.
- Supervision: Kim B, Myung R, Lee MJ, Kim J, Pai H.
- Validation: Kim B, Myung R, Lee MJ, Kim J, Pai H.
- Visualization: Kim B, Myung R.
- Writing - original draft: Kim B.
- Writing - review & editing: Kim B, Myung R, Lee MJ, Pai H.
SUPPLEMENTARY MATERIAL
ICD-10 codes for all-cause pneumonia in the present study
Antibiotic usage pattern for the treatment of community-acquired pneumonia in Korea during 2010–2015 (unit: defined daily dose/episode)
Annual average consumption of antibiotic classes for the treatment of community-acquired pneumonia during 2010–2015.
References
- 1.Kollef MH. Broad-spectrum antimicrobials and the treatment of serious bacterial infections: getting it right up front. Clin Infect Dis. 2008;47(Suppl 1):S3–S13. doi: 10.1086/590061. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States. [Updated 2020]. [Accessed May 18, 2020]. https://www.multivu.com/players/English/8627551-cdc-antibiotic-resistance-threat-report-2019/
- 3.Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(2):155–164. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 4.Choe YJ, Shin JY. Trends in the use of antibiotics among Korean children. Korean J Pediatr. 2019;62(4):113–118. doi: 10.3345/kjp.2018.07290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim B, Hwang H, Kim J, Lee MJ, Pai H. Ten-year trends in antibiotic usage at a tertiary care hospital in Korea, 2004 to 2013. Korean J Intern Med. 2020;35(3):703–713. doi: 10.3904/kjim.2017.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoon YK, Park GC, An H, Chun BC, Sohn JW, Kim MJ. Trends of antibiotic consumption in Korea according to national reimbursement data (2008–2012): a population-based epidemiologic study. Medicine (Baltimore) 2015;94(46):e2100. doi: 10.1097/MD.0000000000002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryu S. The new Korean action plan for containment of antimicrobial resistance. J Glob Antimicrob Resist. 2017;8:70–73. doi: 10.1016/j.jgar.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Lee MS, Oh JY, Kang CI, Kim ES, Park S, Rhee CK, et al. Guideline for antibiotic use in adults with community-acquired pneumonia. Infect Chemother. 2018;50(2):160–198. doi: 10.3947/ic.2018.50.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SH, Song JH, Chung DR, Thamlikitkul V, Yang Y, Wang H, et al. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother. 2012;56(3):1418–1426. doi: 10.1128/AAC.05658-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim T, Park SJ, Chong YP, Park KH, Lee YM, Hong HL, et al. Fluoroquinolone resistance of Streptococcus pneumoniae isolates causing invasive disease: special focus on zabofloxacin. Diagn Microbiol Infect Dis. 2016;86(2):181–183. doi: 10.1016/j.diagmicrobio.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Woodhead M. Pneumonia in the elderly. J Antimicrob Chemother. 1994;34(Suppl A):85–92. doi: 10.1093/jac/34.suppl_a.85. [DOI] [PubMed] [Google Scholar]
- 12.Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong YP, Jung KS, Lee KH, Kim MN, Moon SM, Park S, et al. The bacterial etiology of community-acquired pneumonia in Korea: a nationwide prospective multicenter study. Infect Chemother. 2010;42(6):397. [Google Scholar]
- 14.Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017;32(5):718–728. doi: 10.3346/jkms.2017.32.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNeil SA, Qizilbash N, Ye J, Gray S, Zanotti G, Munson S, et al. A retrospective study of the clinical burden of hospitalized all-cause and pneumococcal pneumonia in Canada. Can Respir J. 2016;2016:3605834. doi: 10.1155/2016/3605834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim B, Myung R, Lee MJ, Kim J, Pai H. Trend of antibiotics usage for acute pyelonephritis in Korea based on national health insurance data 2010–2014. BMC Infect Dis. 2019;19(1):554. doi: 10.1186/s12879-019-4191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venditti M, Falcone M, Corrao S, Licata G, Serra P Study Group of the Italian Society of Internal Medicine. Outcomes of patients hospitalized with community-acquired, health care-associated, and hospital-acquired pneumonia. Ann Intern Med. 2009;150(1):19–26. doi: 10.7326/0003-4819-150-1-200901060-00005. [DOI] [PubMed] [Google Scholar]
- 18.Metersky ML, Tate JP, Fine MJ, Petrillo MK, Meehan TP. Temporal trends in outcomes of older patients with pneumonia. Arch Intern Med. 2000;160(22):3385–3391. doi: 10.1001/archinte.160.22.3385. [DOI] [PubMed] [Google Scholar]
- 19.Wang CC, Lin CH, Lin KY, Chuang YC, Sheng WH. Comparative outcome analysis of penicillin-based versus fluoroquinolone-based antibiotic therapy for community-acquired pneumonia: a nationwide population-based cohort study. Medicine (Baltimore) 2016;95(6):e2763. doi: 10.1097/MD.0000000000002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 21.Organization WH. Definition and general considerations of Defined Daily Dose (DDD) [Accessed May 11, 2020]. http://www.whocc.no/ddd/definition_and_general_considera/
- 22.Seifert H, Blondeau J, Dowzicky MJ. In vitro activity of tigecycline and comparators (2014–2016) among key WHO ‘priority pathogens’ and longitudinal assessment (2004–2016) of antimicrobial resistance: a report from the T.E.S.T. study. Int J Antimicrob Agents. 2018;52(4):474–484. doi: 10.1016/j.ijantimicag.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Song JH, Jung KS, Kang MW, Kim DJ, Pai H, Suh GY, et al. Treatment guidelines for community-acquired pneumonia in Korea: an evidence-based approach to appropriate antimicrobial therapy. Infect Chemother. 2009;41(3):133. [Google Scholar]
- 24.Chang KC, Leung CC, Yew WW, Lau TY, Leung WM, Tam CM, et al. Newer fluoroquinolones for treating respiratory infection: do they mask tuberculosis? Eur Respir J. 2010;35(3):606–613. doi: 10.1183/09031936.00104209. [DOI] [PubMed] [Google Scholar]
- 25.Chen TC, Lu PL, Lin CY, Lin WR, Chen YH. Fluoroquinolones are associated with delayed treatment and resistance in tuberculosis: a systematic review and meta-analysis. Int J Infect Dis. 2011;15(3):e211–6. doi: 10.1016/j.ijid.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Tanne JH. FDA adds “black box” warning label to fluoroquinolone antibiotics. BMJ. 2008;337(7662):a816. doi: 10.1136/bmj.a816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia. an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Solh AA, Sikka P, Ramadan F, Davies J. Etiology of severe pneumonia in the very elderly. Am J Respir Crit Care Med. 2001;163(3 Pt 1):645–651. doi: 10.1164/ajrccm.163.3.2005075. [DOI] [PubMed] [Google Scholar]
- 29.Jeon I, Jung GP, Seo HG, Ryu JS, Han TR, Oh BM. Proportion of aspiration pneumonia cases among patients with community-acquired pneumonia: a single-center study in Korea. Ann Rehabil Med. 2019;43(2):121–128. doi: 10.5535/arm.2019.43.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoon YK, Kim EJ, Chun BC, Eom JS, Park DW, Sohn JW, et al. Prescription of antibiotics for adults hospitalized with community-acquired pneumonia in Korea in 2004: a population-based descriptive study. Respirology. 2012;17(1):172–179. doi: 10.1111/j.1440-1843.2011.02077.x. [DOI] [PubMed] [Google Scholar]
- 31.Kim D, Ahn JY, Lee CH, Jang SJ, Lee H, Yong D, et al. Increasing resistance to extended-spectrum cephalosporins, fluoroquinolone, and carbapenem in gram-negative bacilli and the emergence of carbapenem non-susceptibility in Klebsiella pneumoniae: analysis of Korean antimicrobial resistance monitoring system (KARMS) data from 2013 to 2015. Ann Lab Med. 2017;37(3):231–239. doi: 10.3343/alm.2017.37.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SH, Song SA, Yi J, Song D, Chang CL, Park DC, et al. Distribution and antimicrobial resistance of Streptococcus pneumoniae at four university hospitals in Busan and Gyeongnam. Ann Clin Microbiol. 2016;19(2):48. [Google Scholar]
- 33.Hyun M, Noh CI, Ryu SY, Kim HA. Changing trends in clinical characteristics and antibiotic susceptibility of Klebsiella pneumoniae bacteremia. Korean J Intern Med. 2018;33(3):595–603. doi: 10.3904/kjim.2015.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim YA, Park YS, Youk T, Lee H, Lee K, Lee K. Trends in South Korean antimicrobial use and association with changes in Escherichia coli resistance rates: 12-year ecological study using a nationwide surveillance and antimicrobial prescription database. PLoS One. 2018;13(12):e0209580. doi: 10.1371/journal.pone.0209580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ewig S, Welte T, Chastre J, Torres A. Rethinking the concepts of community-acquired and health-care-associated pneumonia. Lancet Infect Dis. 2010;10(4):279–287. doi: 10.1016/S1473-3099(10)70032-3. [DOI] [PubMed] [Google Scholar]
- 36.Choi MJ, Song JY, Noh JY, Yoon JG, Lee SN, Heo JY, et al. Disease burden of hospitalized community-acquired pneumonia in South Korea: analysis based on age and underlying medical conditions. Medicine (Baltimore) 2017;96(44):e8429. doi: 10.1097/MD.0000000000008429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51–e77. doi: 10.1093/cid/ciw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ICD-10 codes for all-cause pneumonia in the present study
Antibiotic usage pattern for the treatment of community-acquired pneumonia in Korea during 2010–2015 (unit: defined daily dose/episode)
Annual average consumption of antibiotic classes for the treatment of community-acquired pneumonia during 2010–2015.