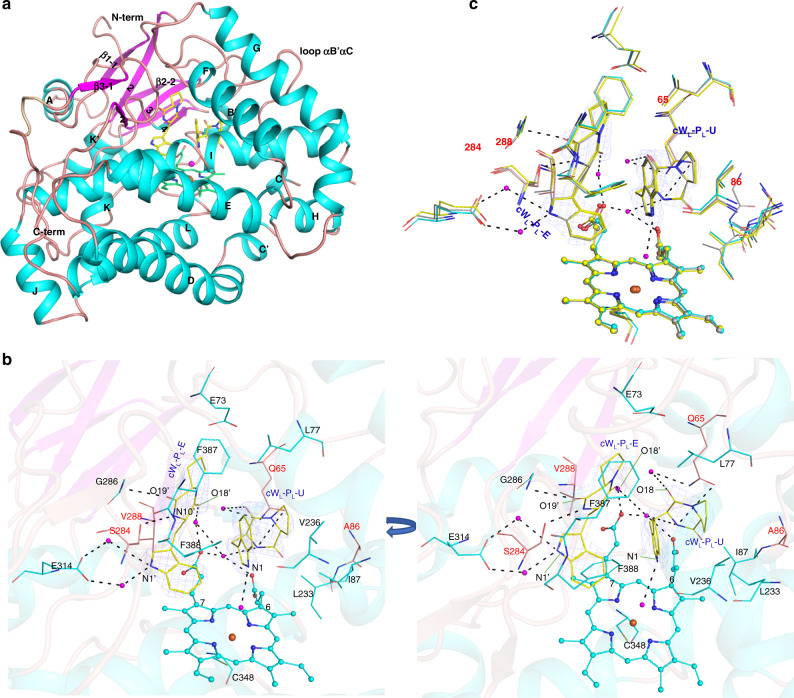
Fig. 3. Crystal structures of NasF5053 and its mutants.
a Cartoon representation of the structure of NasF5053 bound to cWL-PL. Elements of secondary structure and the N/C-termini are labeled; α-helices are shown in cyan and β-strands in magenta. The iron in the heme is shown as a brown sphere and water molecules are displayed as magenta spheres. Other parts of the heme and cWL-PL are displayed as green and yellow sticks, respectively. b A representation of the active site of NasF5053 in complex with cWL-PL-E and cWL-PL-U shown as yellow sticks. Oxygen and nitrogen atoms are shown in red and blue, respectively. The heme is displayed in cyan ball-and-stick representation, with the iron presented as a brown sphere. NasF5053 residues are colored in cyan. Left: “side” view of the active site; right, “top” view. Probable H-bonds between NasF5053, cWL-PL, heme propionate and water molecules (magenta spheres) are indicated as dotted lines. Four critical residues (Q65, A86, S284, and V288) are highlighted in red and shown as orange sticks. c Superposition of the active sites of NasF5053 (cyan sticks), NasF5053-Q65I-A86G (gray sticks), and NasF5053-S84A-V288A (yellow sticks) bound to cWL-PL-E and cWL-PL-U. The locations of four critical residues (65, 86, 284, and 288) are highlighted. The three complex structures are nearly identical, except for the mutated and adjacent residues. The cWL-PL-E and cWL-PL-U substrates in the NasF5053 complex structure are surrounded by Fo–Fc electron density omit map, which is calculated after 20 cycles of refinement in the absence of the ligands and contoured at 2.0 σ level (blue mesh).