Abstract
Alcohol is the most widely used and abused drug among youth in the United States. Teens aged 12–20 years old drink almost 11% of all alcohol consumed in the United States and typically these teens are consuming alcohol in the form of binge drinking. Particularly concerning is that the risk of developing an alcohol use disorder over their lifetime increases the younger one begins to drink. Here we investigated the impact of ethanol drinking in early adolescence on adult ethanol intake using C57BL/6J and DBA/2J mice. We modeled low-dose binge drinking in adolescent mice using a modified Drinking in the Dark (DID) model where the total ethanol intake during adolescence was similar between the strains to specifically ask if low-dose ethanol exposure in the high-alcohol preferring C57BL/6J strain will also lead to increased ethanol intake in adulthood. Our results show that low-dose ethanol drinking in early adolescence dramatically increases adult intake, but only in the alcohol-preferring C57BL/6J strain. Early adolescent ethanol exposure had no effect on ethanol intake in the alcohol-non-preferring DBA/2J mice. These data add to the growing evidence that low-dose ethanol exposures, below the pharmacologically relevant dose, can also contribute to increased drinking in adulthood, but the effect may be influenced by genetic background.
Keywords: adolescence, adulthood, ethanol, mice, DID, intake
Introduction
Alcohol abuse and alcohol use disorder (AUD) is defined as the habitual, compulsive, and uncontrolled consumption of ethanol, coupled with a negative emotional state when not using. AUD is a global health problem resulting in the death of 2.5 million people annually and causes illness and injury to millions more (Organization 2014). In the United States, alcohol use frequently begins during the teenage years and is typically consumed in the form of binge-drinking episodes in adolescents (SAMSHA 2014). Binge drinking is defined as consuming 4–5 alcoholic drinks in a 2 hour period in humans; enough ethanol to achieve blood ethanol levels greater than 80 mg/dl (SAMSHA 2014, (CDC) 2018). Prior experience with ethanol is a strong predictor of alcohol dependence later in life (Grant and Dawson 1997). The rate of lifetime alcohol dependence reached 40% when drinking began before age 14, while lifetime alcohol dependence was limited to 10% in individuals who started drinking after age 20 (Grant and Dawson 1997).
Excessive ethanol consumption during adolescence can be particularly devastating as it may delay or disrupt critical neurodevelopmental processes, leading to profound consequences in adult brain structure, connection, and function with some behavioral effects persisting into adulthood (Crews et al. 2000, Spear 2000). For example in rodent models, adolescent ethanol exposure increases ethanol intake (Moore et al. 2010, Strong et al. 2010, Alaux-Cantin et al. 2013) and risk-taking preference (Spear 2000), and impairs conditioned discrimination, memory (Pascual et al. 2007, Montesinos et al. 2014, Wolstenholme et al. 2017) and reversal learning (Coleman et al. 2011, Coleman et al. 2014) in adult animals. These impairments were more substantial when ethanol exposure occurred during adolescence rather than in adulthood (White et al. 2000, Sircar and Sircar 2005, Bergstrom et al. 2006). Furthermore, binge ethanol exposure in adolescence increased ethanol behavioral sensitivity and decreased ethanol’s aversive effects, which can contribute to increased drinking at a young age (Spear 2000). In general, adolescent mice (Ho et al. 1989, Tambour et al. 2008, Strong et al. 2010, Metten et al. 2011) and rats (Bergstrom et al. 2006, Maldonado-Devincci et al. 2010) tend to drink more ethanol than adults on a gram per kilogram basis, and this increase sometimes persists into adulthood. More importantly, adult mice exposed to ethanol as juveniles (~PND 21 to 42) tend to drink more of an ethanol solution than adults given first access during adulthood (Ho et al. 1989, Blizard et al. 2004, Lee et al. 2017). Although several studies have found no effect of age on ethanol consumption. For example, adolescent rats (Siegmund et al. 2005, Bell et al. 2011) and C57BL/6J mice (Hefner and Holmes 2007) did not display increased drinking in adulthood. This however, may be genotype-dependent as Balb/cJ (Blizard et al. 2004) and DBA/2J inbred strains (Moore et al. 2010) and HDID mice, selectively bred for high ethanol consumption (Metten et al. 2011), also did not display increased drinking following adolescent exposure.
In the present study, we modeled voluntary low-dose drinking during adolescence in two inbred strains, C57BL/6J and DBA/2J, known for their divergent behavioral responses to ethanol. For example, adult DBA/2J mice have larger locomotor activation and sedative/hypnotic response to acute ethanol and drink very little ethanol, while C57BL/6J mice are the opposite (Phillips et al. 1994, Crabbe et al. 1999). As with adults, C57BL/6J adolescents consume more ethanol than DBA/2J using a drinking in the dark (DID) paradigm (Moore et al. 2010). When retested as adults, C57BL/6J mice exposed to DID during adolescence drank more ethanol than mice previously exposed as adults; DBA/2J mice still did not increase their drinking, as others have reported (Moore et al. 2010). However, as noted by the authors, an important caveat of this study is that the DBA/2J and C57BL/6J mice consumed different amount of alcohol during adolescence (Moore et al. 2010). We have previously shown that C57BL/6J mice offered 20% (v/v) ethanol solutions with a modified DID protocol increased both DID and 2-bottle choice drinking in adulthood (Younis et al. 2019). Here we asked the question, does adolescent exposure to ethanol in the binge drinking DID model also increase drinking in adulthood in these two inbred mouse strains? In order to directly compare the C57BL/6J and DBA/2J strains, we titrated the concentration of ethanol solutions offered to both C57BL/6J and DBA/2J mice so both strains voluntarily consumed similar amounts of total ethanol and had similar blood ethanol concentrations during the 4-hour access period.
Materials and Methods
Animals
Adolescent male C57BL/6J and DBA/2J mice from Jackson Laboratories (Bar Harbor, ME, USA) arrived at PND 21 and were housed individually in standard Plexiglas cages (12×18×28 cm) with corn-cob bedding in a temperature and humidity controlled room (22±2°C, 50±5%) under a 12-hour inverted light/dark cycle (lights on at 6PM). Mice had food (Teklad, Envigo, Madison, WI; cat. #7012) and water available ad libitum, except where noted during DID sessions. Cages contained small cardboard tubes and a cotton nestlet (5×5 cm) for the purpose of environmental enrichment. Mice were acclimated to the vivarium for one week while water intake and body weight were measured. On PND 28, three hours into the dark cycle, adolescent mice began the DID protocol. Institutional Animal Care and Use Committee of Virginia Commonwealth University approved the study. All studies were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Phase I: Drinking in the dark (DID) during adolescence
We used a slightly modified mouse DID model (Rhodes et al. 2005, Dawson et al. 2018, Younis et al. 2019). Male C57BL/6J and DBA/2J adolescent mice (n=8/group) were exposed to one of two single-bottle (25 ml sipper tubes) conditions for a 4-hour period from PND 28 to PND 36 beginning 3 hours after lights out: (1) nine days consecutive daily access to 5% (v/v for C57BL/6J) and 20% (v/v for DBA/2J) ethanol in tap water or (2) tap water only. In all cases, after the 4-hour period, bottles were replaced with water-only bottles. On day nine, immediately after measuring ethanol intake, blood samples were collected via submandibular bleed. Mice remained singly housed for 36 days with access ad libitum to food and water until they reached adulthood.
Phase II: Drinking in the dark (DID) during adulthood
To determine the impact of early exposure on adult ethanol consumption, all groups of mice from Phase I were exposed to ethanol daily from PND 72 to PND 80. As during adolescence, C57BL/6J mice received 5% (v/v) and DBA/2J received 20% (v/v). Three hours after lights out, 25 ml water tubes were replaced with sipper tubes containing ethanol in tap water and mice were allowed to drink for 4 hours for nine consecutive days. In both phases, spillage and evaporation were accounted for by placing ethanol tubes into an empty cage, without mice. The average value of ethanol missing from these control tubes was subtracted from individual drinking values for each day.
Blood Ethanol Concentration (BEC) Determination
Blood was collected via submandibular bleed using 5 mm Lancets (Medipoint, Inc., Minenola, NY) and stored in heparinized BD microtainers (Elkins-Sinn, Inc., Cherry Hill, NJ). Blood was analyzed for ethanol content using gas chromatography similar to a previously described procedure (Gallaher et al. 1996, Neddenriep et al. 2019, Younis et al. 2019). Animals were injected with saline (s.c) to avoid hypovolemia and received food/water ad libitum after blood collection.
Statistical Analysis
The data were analyzed using the GraphPad Prism software, version 8.1.2 (GraphPad Software, Inc., La Jolla, CA) and are expressed as the mean ± S.E.M. Statistical analysis was performed using two-way repeated measures analysis of variance (RM ANOVA) for average daily ethanol intake in C57BL/6J and DBA/2J mice. An overall three-way repeated measure ANOVA was applied to determine the main interaction between three factors: strain x treatment x time. Two-way ANOVA was used to compare adult average ethanol intake with time and ethanol pre-treatment as factors. The Holm-Sidak post hoc multiple comparisons test was used to determine the difference between treatment groups and at multiple time points, as appropriate. Unpaired, two-tailed student’s t-tests were used to compare the adolescent average daily ethanol intake and average blood ethanol concentration. Comparisons were considered statistically significant when p < 0.05.
Results
The impact of mouse strain on the rate of ethanol consumption in adolescence
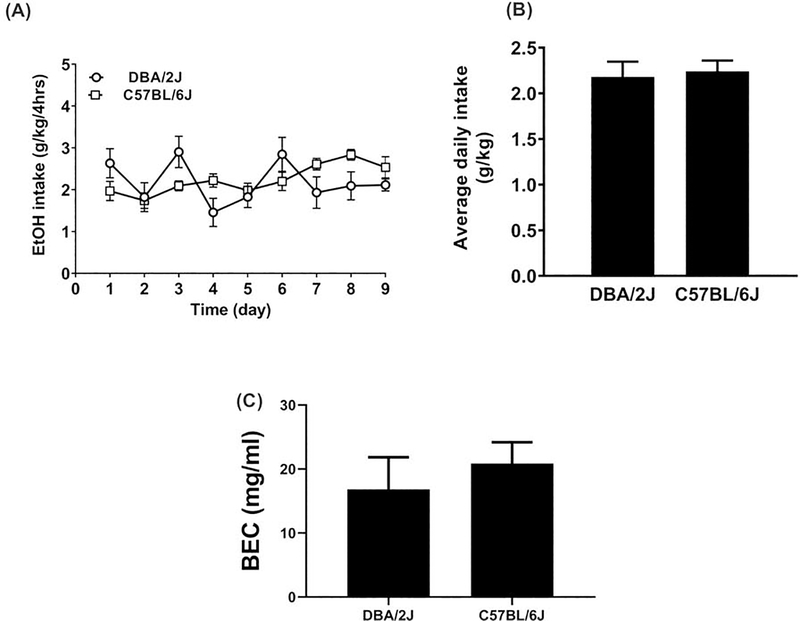
Our objective was to reduce the total ethanol intake of C57BL/6J mice to the level of DBA/2J mice to ask the question whether low levels of ethanol exposure would also lead to increased ethanol consumption in adulthood. We first investigated whether ethanol intake would differ between the alcohol-preferring C57BL/6J strain at 5% concentration and the non-alcohol preferring DBA/2J strain at 20% concentration during adolescence. Two-way RM ANOVA showed no significant difference in daily ethanol intake between the two strains (Fstrain (1, 14) = 0.13, P=0.72) during adolescence (Figure 1A). Although, two-way RM ANOVA revealed a significant main effect of time (Ftime (8, 112) = 2.6; P<0.01) and a significant interaction between strain and time (Ftime x strain (8, 112) = 3.2; P=0.003). Additionally, the two strains of mice did not differ in their average total ethanol intake during the adolescent period (t=0.30, df=16; P=0.77, Figure 1B) or in their blood-ethanol concentration on the last day of ethanol access (t=0.69, df= 11; P=0.50, Figure 1C).
Figure 1: Daily ethanol intake in adolescent male C57BL/6J and DBA/2J mice.
A. Daily ethanol (EtOH) intake in grams per kilogram body weight during adolescent exposure (PND28 to PND36) to 5% for C57BL/6J and 20% for DBA/2J ethanol using a modified DID procedure for 9 days. B. Average ethanol intake over the 9 day adolescent period. C. Average blood ethanol content (BEC) for C57BL/6J and DBA/2J mice on day 9 immediately following DID access at PND 36. Each point represents the mean ± SEM of 8 animals per group and genotype.
Adolescent binge drinking enhances ethanol consumption in adulthood
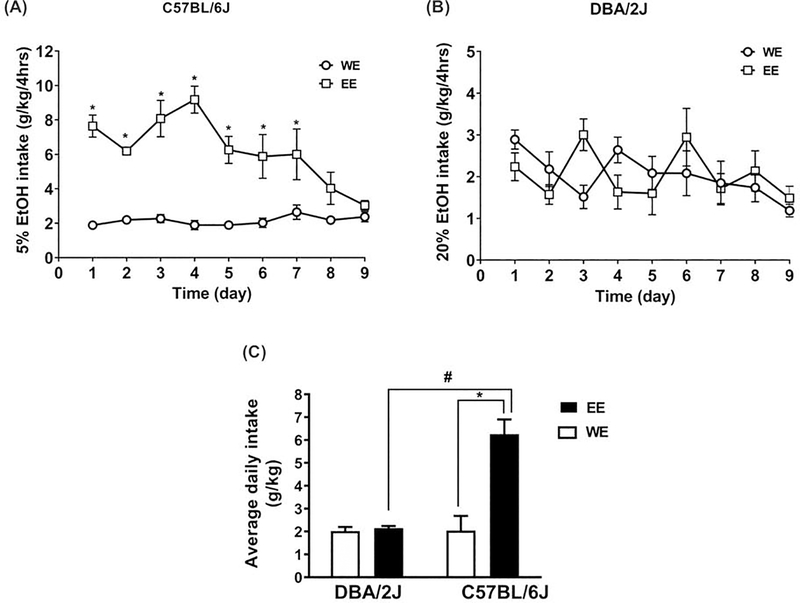
We then examined the impact of adolescent (PND28-PND36) ethanol DID drinking on later adult ethanol consumption (PND72-PND80) in the two strains of mice. A three-way repeated measures ANOVA revealed a significant interaction between the two different strains (C57BL/6J, DBA/2J), treatments (water and ethanol), and time (Ftime x strain x treatment (8,224)=5.5, P<0.0001). A separate two-way RM ANOVAs were also performed within each strain to assess strain-dependent differences in intake. As seen in Figure 2A, two-way RM ANOVA revealed a significant interaction between the time and treatment in the C57BL/6J adult mice who were exposed to 5% ethanol versus water in adolescence (Ftime x treatment (8, 112) = 5.13; P<0.0001). A significant main effect of time (Ftime (8,112) = 4.05; P=0.0003) and treatment (Ftreatment (1, 14) = 92.82; P<0.0001) was also noted, where C57BL/6J mice with prior ethanol drinking in adolescence increased adult ethanol intake. In DBA/2J mice, a main effect of time (Ftime (8, 112) = 2.50; P<0.016) and an interaction between time and treatment (Ftime x treatment (8, 112) = 2.8; P=0.008) was noted in adolescence. No significant effect of adolescent drinking was seen in adult ethanol intake levels (Figure 2B). No main effect of treatment (Ftreatment (1, 14) = 0.003; P=0.96) was found in DBA/2J mice. When comparing between strains for the average ethanol intake during the adult access period (Figure 2C), C57BL/6J mice with an adolescent history of low dose drinking had significantly increased average ethanol intake during adulthood as compared to ethanol naïve C57BL/6J adult mice or DBA/2J mice regardless of drinking history (Fstrainx treatment (1,32) = 34.44, P<0.0001). A main effect of adolescent exposure (Ftreatment (1,32) = 39.07; P<0.0001) and time (Ftime (1, 32) = 35.04, P<0.0001) were also found (Figure 2C).
Figure 2: Ethanol drinking in adolescence increased ethanol intake later in life in C57BL/6J but not DBA/2J mice.
Mice exposed to DID with ethanol (EE) vs. water only (WE) during adolescence showed higher daily ethanol (EtOH) intake profiles in the adult phase of exposure (PND72 to PND80) in C57BL/6J but not DBA/2J mice. C57BL/6J had access to 5% ethanol while DBA/2J had access to 20% ethanol. Adult daily ethanol intake (g/kg/4 hours) from PND 72–80 in a daily exposure modified DID procedure in A. C57BL/6J and B. in DBA/2J mice. C. Average ethanol intake in C57BL/6J and DBA/2J males for the 9 day period. *Denotes p<0.05 vs. WE group. #Denotes DBA/2J EE vs C57Bl/6J EE. Each point represents the mean ± SEM of 8 animals per group and genotype.
Discussion
The aim of this study was to model voluntary low-dose binge ethanol drinking in adolescent C57BL/6J and DBA/6J mice and to determine if a history of drinking would increase adult ethanol consumption. We hypothesized that low-dose drinking in the DID paradigm during adolescence would increase ethanol intake in adulthood in C57BL/6J but not DBA/2J males when compared to animals who are first exposed to ethanol in adulthood. To model binge drinking in both strains, we used a modified DID protocol where C57BL/6J mice were offered 5% (v/v) ethanol and DBA/2J mice were offered 20% (v/v) ethanol. Both C57BL/6J and DBA/2J mice consumed similar amounts of ethanol (~2.2 g/kg) during the adolescent period when the ethanol solutions were offered at these concentrations. C57BL/6J mice dramatically increased their ethanol intake to 8 g/kg ethanol when re-introduced to the ethanol DID paradigm in adulthood (PND 72–80). In contrast, C57BL/6J mice that were only given ethanol DID in adulthood consumed ~2 g/kg of the 5% (v/v) ethanol solution. DBA/2J mice did not increase their ethanol intake in adulthood regardless of whether they had ethanol access as adolescents.
One question that has been unanswered in the literature is if the dose of ethanol consumed in adolescence matters for these subjects to increase drinking in adulthood. Using a scheduled high alcohol consumption (SHAC) protocol offering 5% ethanol to establish pharmacological levels of binge drinking during adolescence and then offering increasing ethanol solutions in adulthood, both adolescent C57BL/6J males and females with a history of binge drinking increased ethanol intake and preference for a 5% ethanol solution when offered for 24 hours as compared to ethanol naive mice (Strong et al. 2010). Females, but not males, with a binge ethanol history also increased 10% ethanol intake in adulthood, while 20% ethanol intake or preference were not different in adulthood following adolescent binge ethanol (Strong et al. 2010). When ethanol was offered in a shorter 2-hour binge, only 20% ethanol preference was increased in adulthood; 10% or 20% ethanol intake were not increased in adults following adolescent binge ethanol (Strong et al. 2010). In most other studies, adolescent mice are usually offered high concentration (i.e. 10% or 20%) ethanol solutions in order to induce binge levels of intoxication (Ho et al. 1989, Blizard et al. 2004, Moore et al. 2010, Metten et al. 2011) and these usually increase drinking in adulthood, although sometimes only modestly. We recently reported that C57BL/6J adolescents offered 20% ethanol consumed ~7 g/kg ethanol in a four-hour period on average with corresponding BECs to be >80 mg/dL (Younis et al. 2019), reaching a “pharmacologically reinforcing” level (Heilig and Koob 2007). As adults, these mice increased both ethanol DID and 2 bottle choice voluntary drinking relative to mice with no pre-exposure to ethanol (Younis et al. 2019). However, the relative increase in adult ethanol intake from adolescent intake during the DID paradigm was not very large since mice increased from ~7 to only ~8 g/kg. These data suggest that C57BL/6J mice may have already reached an upper limit for the amount of ethanol they will consume in a four-hour period, and thus we may have observed a ceiling effect on adult intake limits. In the present study, C57BL/6J mice were offered 5% ethanol and only consumed ~2 g/kg in a four-hour period during adolescence. Blood ethanol concentrations were also low (~20 mg/dL), but detectable (Figure 1). These C57BL/6J mice dramatically increased their ethanol intake four-fold when the ethanol DID paradigm was re-introduced in adulthood. Mice resumed drinking at 8 g/kg in a four-hour period and this continued for the first four days of access before slowly decreasing to ~3g/kg over the 9-day access period. These data suggest that even low levels of ethanol consumption in adolescence can increase risk for high levels of adult drinking. However, as we and others have noted, the pattern and dose of ethanol exposure in adolescence influences the extent of adult increases in drinking. Low-dose ethanol drinking may also influence the transient nature of the observed adult increases in drinking, and may be enhancing the alcohol deprivation effect. Further studies will be needed to investigate the effects of low-dose drinking on both the alcohol deprivation effect and transient nature of increased drinking. However, if these patterns of drinking continue following periods of abstinence and access, such individuals could be at substantially higher risk for developing an alcohol use disorder.
Other studies have shown similar age-related effects in adult drinking following ethanol pre-exposure in adolescence. In the genetically heterogeneous WSC-1 mice, derived from an 8-way cross of inbred mice, animals first exposed to ethanol 2-bottle choice drinking in adolescence (i.e. at 4 weeks) showed a transient increase in ethanol intake as compared to their adult counterparts, first given ethanol 2-bottle choice at 10 weeks (Tambour et al. 2008). However, by the end of the 8-week continuous ethanol access period, when all mice were adults, animals first exposed to ethanol during adolescence or adulthood no longer differed in their ethanol intake levels (Tambour et al. 2008). Using the HDID strain, mice selectively bred for high blood ethanol after DID, Metten et al. (2011) showed that HDID mice given ethanol access beginning at 4 weeks generally drank more ethanol than those of other age groups (i.e. 6 and 9 weeks old). When comparing mice at the same ages, 9-week old HDID mice initiated at 4 weeks drank more ethanol than did naïve 9-week olds, but once the mice reached 10 weeks of age, all three groups of age-matched mice drank equivalent amounts. Thus, it appears in our current study and in those of others (Tambour et al. 2008, Metten et al. 2011), that the DID test is a sensitive test that can detect differences in ethanol intake relative to developmental age.
Of note, in this and other cited studies, animals are single housed for varying periods of time prior to and during the drinking sessions. Single housing in adolescence can be particularly detrimental to proper neurodevelopment and function (Arain et al. 2013, Butler et al. 2016) and increases risk for alcohol use and abuse (Lopez et al. 2011, Sanna et al. 2011 ). Adolescents are particularly vulnerable to social isolation stress as compared to adults and thus single housing is frequently used to model post-traumatic stress (Skelly et al. 2015, Gilpin and Weiner 2017), depression, and anxiety (Koike et al. 2009, Amiri et al. 2015). In the few studies that have directly investigated drinking behavior in single versus group housed rodents, social isolation usually increases ethanol consumption in rats (Schenk et al. 1990, Wolffgramm 1990, Chappell et al. 2013, Skelly et al. 2015) and mice (Advani et al. 2007, Lopez et al. 2011, Sanna et al. 2011). These effects appear to be limited to adolescence, as increased ethanol preference does not usually occur in isolated adult rodents (Schenk et al. 1990, Lopez et al. 2011, Chappell et al. 2013) suggesting that early exposure to social isolation may interact with developmental processes and contribute to ethanol sensitivity later in adult life. The social behavior circuits may be uniquely altered by ethanol’s actions in adolescence; however, this is not addressed in the current study.
Notably in the present study, DBA/2J mice did not change their ethanol intake patterns in adolescence or adulthood; as has been reported previously with 2-bottle choice drinking (Moore et al. 2010). DBA/2J mice given 20% ethanol DID in adolescence consumed ~2 g/kg ethanol in adolescence and in adulthood. This level was not altered by ethanol pre-exposure in adolescence (Figure 2). It is important to note that the average daily ethanol intake during adolescence was similar between C57BL/6J and DBA/2J males. Blood ethanol concentrations were also low and did not differ between the strains, suggesting that both strains consumed ethanol during the DID sessions and their ethanol metabolism likely did not differ. Thus, it is unlikely that DBA/2J mice did not increase drinking because their exposure level to ethanol was too low. Rather, DBA/2J mice may primarily be avoiding the ethanol due to strain-dependent taste preferences or due to the aversive taste of higher percent ethanol. Indeed, a caveat to the present study is that DBA/2J and C57BL/6J mice were given different concentrations of ethanol in an attempt to reduce a potential ceiling effect in C57BL/6J mice. However, others have noted shifts in taste perception of ethanol when different concentrations are used (Bachmanov et al. 1996). For example, a 5% ethanol solution may be perceived as less aversive than a 20% ethanol solution since ethanol intake and preference in C57BL/6J mice increased with increasing ethanol concentrations to peak at 10% ethanol and then decreased (Bachmanov et al. 1996) when mice were offered ethanol solutions of 1, 5 10, 15 and 20% ethanol. It is possible that the low ethanol consumption in the DBA/2J mice in this study could be due to aversive issues in smell and/or taste perception of the higher 20% ethanol solution and thus revealing a “floor effect” in DBA/2J mice. Thus, our study has a confound of differing taste perceptions of ethanol due to the concentrations offered. Both C57BL/6J and DBA/2J mice will readily self-administer ethanol intravenously (Grahame and Cunningham 1997), or intragastrically (Fidler et al. 2011, Fidler et al. 2012), bypassing the aversive issues associated with ethanol taste or odor. Indeed, DBA/2J mice have polymorphisms in the Tas1r3 gene, a “sweet” taste receptor which confers a reduced response to sweet compounds (Max et al. 2001, Montmayeur et al. 2001), and may contribute to the lower ethanol intakes in DBA/2J mice, especially at the higher ethanol concentrations. Addition of monosodium glutamate (MSG), but not sucrose, to ethanol solutions increased ethanol DID and 2-bottle choice drinking in DBA/2J mice and this continued after the MSG was faded out of the ethanol solutions (McCool and Chappell 2012, 2014). Thus, the strain-dependent effects of adolescent drinking on future adult intake in the current study are likely due to strain dependent differences in ethanol taste perception. Although, genetic differences in sensitivity to ethanol’s aversive post-absorptive effects may still play a role in determining strain differences in ethanol drinking (Cunningham 2019).
Studies on whether adolescent exposure to ethanol reliably increases ethanol intake in adulthood have given mixed results. But a few generalizations can be made. For example, increased ethanol consumption in adult mice with prior ethanol experience is influenced by strain (Blizard et al. 2004, Moore et al. 2010) and usually more reliable in females (Strong et al. 2010). In general, C57BL/6J males given initial access to ethanol beginning at 3 weeks (Ho et al. 1989), or at 4 weeks of age (Moore et al. 2010, Metten et al. 2011) tended to consume more ethanol as adults than mice without prior ethanol experience. The ethanol access paradigm used in either study (2-bottle choice in (Ho et al. 1989) and DID in (Moore et al. 2010)) did not have a large effect as either paradigm increased adult drinking when adults were given ethanol access as adolescents. Non-C57BL/6J strains, such as the heterogeneous stock, HS/Npt (Metten et al. 2011), and Balb/cByJ (Blizard et al. 2004), also increased ethanol intake as adults when given adolescent DID ethanol access. However, other strains given free-choice access (Balb/cJ; (Blizard et al. 2004)) and/or forced (single tube) access (DBA/2J; (Moore et al. 2010) and HDID (Metten et al. 2011)) did not show adult increases in ethanol intake following adolescent pre-exposure. One study using daily 2-bottle choice ethanol access for a 3-week period in adolescence beginning at either 4, 5, or 6 weeks of age in C57BL/6J males did not show an increase in adult drinking after 4 weeks of abstinence (Hefner and Holmes 2007). Thus, genetic differences in risk for adult drinking following adolescent pre-exposure cannot be ruled out or simply explained away by strain-dependent differences in taste perception.
In summary, we show that low-dose ethanol drinking in C57BL/6J mice dramatically increases ethanol intake in adulthood. The magnitude of this effect was large, suggesting that other studies using ethanol DID in C57BL/6J mice may suffer from a potential ceiling effect when using high dose ethanol solutions. Further, our findings offer potential evidence that even low levels of ethanol exposure during the adolescent period could increase risk for adult alcohol abuse potential. Further studies are needed to expand these finding into females and to identify the molecular signaling pathways that are uniquely activated by low-dose ethanol in the adolescent brain.
Highlights.
Ethanol solutions were titrated so C57BL/6J mice consumed similar amounts as DBA/2J.
Adolescent blood ethanol concentrations were similar between C57BL/6J and DBA/2J mice.
Adolescent C57BL/6J mice increased ethanol intake in adulthood; DBA/2J mice did not.
Low-dose ethanol drinking in adolescence can dramatically increase adult drinking.
Acknowledgments
This work was supported by NIAAA [grant number P50AA022537] and NIDA [grant number P30DA033934].
Abbreviations
- AUD
alcohol use disorder
- DID
drinking in the dark
- BEC
blood ethanol concentration
- PND
postnatal day
- EE
ethanol exposed
- WE
water exposed
Footnotes
Declaration of Interest: The authors have no competing interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (CDC), C. f. D. C. a. P. (2018, August 2, 2018). “Fact Sheets - Underage Drinking.” Retrieved September 30, 2019, from http://www.cdc.gov/alcohol/fact-sheets/underage-drinking.htm.
- Advani T, Hensler JG and Koek W (2007). “Effect of early rearing conditions on alcohol drinking and 5-HT1A receptor function in C57BL/6J mice.” Int J Neuropsychopharmacol 10(5): 595–607. https://10.1017/S1461145706007401 [DOI] [PubMed] [Google Scholar]
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C and Naassila M (2013). “Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens.” Neuropharmacology 67: 521–531. https://10.1016/j.neuropharm.2012.12.007 [DOI] [PubMed] [Google Scholar]
- Amiri S, Haj-Mirzaian A, Rahimi-Balaei M, Razmi A, Kordjazy N, Shirzadian A, Ejtemaei Mehr S, Sianati H and Dehpour AR (2015). “Co-occurrence of anxiety and depressive-like behaviors following adolescent social isolation in male mice; possible role of nitrergic system.” Physiol Behav 145: 38–44. https://10.1016/j.physbeh.2015.03.032 [DOI] [PubMed] [Google Scholar]
- Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, Sandhu R and Sharma S (2013). “Maturation of the adolescent brain.” Neuropsychiatr Dis Treat 9: 449–461. https://10.2147/NDT.S39776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG and Beauchamp GK (1996). “Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice.” Alcohol Clin Exp Res 20(2): 201–206. https://10.530-0277.1996.tb01630.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Smith RJ, Toalston JE, Franklin KM and McBride WJ (2011). “Modeling binge-like ethanol drinking by peri-adolescent and adult P rats.” Pharmacol Biochem Behav 100(1): 90–97. https://10.1016/j.pbb.2011.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom HC, McDonald CG and Smith RF (2006). “Alcohol exposure during adolescence impairs auditory fear conditioning in adult Long-Evans rats.” Physiol Behav 88(4–5): 466–472. https://10.1016/j.physbeh.2006.04.021 [DOI] [PubMed] [Google Scholar]
- Blizard D6A, Vandenbergh DJ, Jefferson AL, Chatlos CD, Vogler GP and McClearn GE (2004). “Effects of periadolescent ethanol exposure on alcohol preference in two BALB substrains.” Alcohol 34(2–3): 177–185. https://10.1016/j.alcohol.2004.08.007 [DOI] [PubMed] [Google Scholar]
- Butler TR, Karkhanis AN, Jones SR and Weiner JL (2016). “Adolescent Social Isolation as a Model of Heightened Vulnerability to Comorbid Alcoholism and Anxiety Disorders.” Alcohol Clin Exp Res 40(6): 1202–1214. https://10.1111/acer.13075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell AM, Carter E, McCool BA and Weiner JL (2013). “Adolescent rearing conditions influence the relationship between initial anxiety-like behavior and ethanol drinking in male Long Evans rats.” Alcohol Clin Exp Res 37 Suppl 1: E394–403. https://10.1111/j.1530-0277.2012.01926.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG Jr., He J, Lee J, Styner M and Crews FT (2011). “Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice.” Alcohol Clin Exp Res 35(4): 671–688. https://10.1111/j.1530-0277.2010.01385.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG Jr., Liu W, Oguz I, Styner M and Crews FT (2014). “Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility.” Pharmacol Biochem Behav 116: 142–151. https://10.1016/j.pbb.2013.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D and Dudek BC (1999). “Genetics of mouse behavior: interactions with laboratory environment.” Science 284(5420): 1670–1672. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC and Knapp DJ (2000). “Binge Ethanol Consumption Causes Differential Brain Damage in Young Adolescent Rats Compared With Adult Rats.” Alcoholism: Clinical and Experimental Research 24(11): 1712–1723. [PubMed] [Google Scholar]
- Cunningham CL (2019). “Genetic Relationships Between Ethanol-Induced Conditioned Place Aversion and Other Ethanol Phenotypes in 15 Inbred Mouse Strains.” Brain Sci 9(8). https://10.3390/brainsci9080209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A, Wolstenholme JT, Roni MA, Campbell VC, Jackson A, Slater C, Bagdas D, Perez EE, Bettinger JC, De Biasi M, Miles MF and Damaj MI (2018). “Knockout of alpha 5 nicotinic acetylcholine receptors subunit alters ethanol-mediated behavioral effects and reward in mice.” Neuropharmacology 138: 341–348. https://10.1016/j.neuropharm.2018.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler TL, Dion AM, Powers MS, Ramirez JJ, Mulgrew JA, Smitasin PJ, Crane AT and Cunningham CL (2011). “Intragastric self-infusion of ethanol in high- and low-drinking mouse genotypes after passive ethanol exposure.” Genes Brain Behav 10(3): 264–275. https://10.1111/j.1601-183X.2010.00664.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler TL, Powers MS, Ramirez JJ, Crane A, Mulgrew J, Smitasin P and Cunningham CL (2012). “Dependence induced increases in intragastric alcohol consumption in mice.” Addict Biol 17(1): 13–32. https://10.1111/j.1369-1600.2011.00363.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher EJ, Jones GE, Belknap JK and Crabbe JC (1996). “Identification of genetic markers for initial sensitivity and rapid tolerance to ethanol-induced ataxia using quantitative trait locus analysis in BXD recombinant inbred mice.” J Pharmacol Exp Ther 277(2): 604–612. [PubMed] [Google Scholar]
- Gilpin NW and Weiner JL (2017). “Neurobiology of comorbid post-traumatic stress disorder and alcohol-use disorder.” Genes Brain Behav 16(1): 15–43. https://10.1111/gbb.12349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame NJ and Cunningham CL (1997). “Intravenous ethanol self-administration in C57BL/6J and DBA/2J mice.” Alcohol Clin Exp Res 21(1): 56–62. [PubMed] [Google Scholar]
- Grant BF and Dawson DA (1997). “Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey.” J Subst Abuse 9: 103–110. [DOI] [PubMed] [Google Scholar]
- Hefner K and Holmes A (2007). “An investigation of the behavioral actions of ethanol across adolescence in mice.” Psychopharmacology (Berl) 191(2): 311–322. https://10.1007/s00213-006-0646-2 [DOI] [PubMed] [Google Scholar]
- Heilig M and Koob GF (2007). “A key role for corticotropin-releasing factor in alcohol dependence.” Trends Neurosci 30(8): 399–406. https://10.1016/j.tins.2007.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A, Chin AJ and Dole VP (1989). “Early experience and the consumption of alcohol by adult C57BL/6J mice.” Alcohol 6(6): 511–515. https://10.1016/0741-8329(89)90060-8 [DOI] [PubMed] [Google Scholar]
- Koike H, Ibi D, Mizoguchi H, Nagai T, Nitta A, Takuma K, Nabeshima T, Yoneda Y and Yamada K (2009). “Behavioral abnormality and pharmacologic response in social isolation-reared mice.” Behav Brain Res 202(1): 114–121. https://10.1016/j.bbr.2009.03.028 [DOI] [PubMed] [Google Scholar]
- Lee KM, Coehlo MA, Solton NR and Szumlinski KK (2017). “Negative Affect and Excessive Alcohol Intake Incubate during Protracted Withdrawal from Binge-Drinking in Adolescent, But Not Adult, Mice.” Front Psychol 8: 1128 https://10.3389/fpsyg.2017.01128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Doremus-Fitzwater TL and Becker HC (2011). “Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6J mice.” Alcohol 45(4): 355–364. https://10.1016/j.alcohol.2010.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Devincci AM, Alipour KK, Michael LA and Kirstein CL (2010). “Repeated binge ethanol administration during adolescence enhances voluntary sweetened ethanol intake in young adulthood in male and female rats.” Pharmacol Biochem Behav 96(4): 476–487. https://10.1016/j.pbb.2010.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S and Margolskee RF (2001). “Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac.” Nat Genet 28(1): 58–63. https://10.1038/88270 [DOI] [PubMed] [Google Scholar]
- McCool BA and Chappell AM (2012). “Using monosodium glutamate to initiate ethanol self-administration in inbred mouse strains.” Addict Biol 17(1): 121–131. https://10.1111/j.1369-1600.2010.00260.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA and Chappell AM (2014). “Persistent enhancement of ethanol drinking following a monosodium glutamate-substitution procedure in C57BL6/J and DBA/2J mice.” Alcohol 48(1): 55–61. https://10.1016/j.alcohol.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metten P, Brown LL and Crabbe JC (2011). “Limited access ethanol drinking in the dark in adolescent and adult mice.” Pharmacol Biochem Behav 98(2): 279–285. https://10.1016/j.pbb.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos J, Pascual M, Pla A, Maldonado C, Rodriguez-Arias M, Minarro J and Guerri C (2014). “TLR4 elimination prevents synaptic and myelin alterations and long-term cognitive dysfunctions in adolescent mice with intermittent ethanol treatment.” Brain Behav Immun. https://10.1016/j.bbi.2014.11.015 [DOI] [PubMed] [Google Scholar]
- Montmayeur JP, Liberles SD, Matsunami H and Buck LB (2001). “A candidate taste receptor gene near a sweet taste locus.” Nat Neurosci 4(5): 492–498. https://10.1038/87440 [DOI] [PubMed] [Google Scholar]
- Moore EM, Mariani JN, Linsenbardt DN, Melon LC and Boehm SL 2nd (2010). “Adolescent C57BL/6J (but not DBA/2J) mice consume greater amounts of limited-access ethanol compared to adults and display continued elevated ethanol intake into adulthood.” Alcohol Clin Exp Res 34(4): 734–742. https://10.1111/j.1530-0277.2009.01143.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neddenriep B, Bagdas D, Contreras KM, Ditre JW, Wolstenholme JT, Miles MF and Damaj MI (2019). “Pharmacological mechanisms of alcohol analgesic-like properties in mouse models of acute and chronic pain.” Neuropharmacology: 107793 https://10.1016/j.neuropharm.2019.107793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization, W. H. (2014). Global status report on alcohol and health 2014..
- Pascual M, Blanco AM, Cauli O, Minarro J and Guerri C (2007). “Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats.” Eur J Neurosci 25(2): 541–550. https://10.1111/j.1460-9568.2006.05298.x [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Dickinson S and Burkhart-Kasch S (1994). “Behavioral sensitization to drug stimulant effects in C57BL/6J and DBA/2J inbred mice.” Behav Neurosci 108(4): 789–803. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA and Crabbe JC (2005). “Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice.” Physiol Behav 84(1): 53–63. https://10.1016/j.physbeh.2004.10.007 [DOI] [PubMed] [Google Scholar]
- SAMSHA (2014). Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD, Substance Abuse and Mental Health Services Administration; NSDUH Series H-48. [Google Scholar]
- Sanna E, Talani G, Obili N, Mascia MP, Mostallino MC, Secci PP, Pisu MG, Biggio F, Utzeri C, Olla P, Biggio G and Follesa P (2011). “Voluntary Ethanol Consumption Induced by Social Isolation Reverses the Increase of alpha(4)/delta GaBa(A) Receptor Gene Expression and Function in the Hippocampus of C57BL/6J Mice.” Front Neurosci 5: 15 https://10.3389/fnins.2011.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Gorman K and Amit Z (1990). “Age-dependent effects of isolation housing on the self-administration of ethanol in laboratory rats.” Alcohol 7(4): 321–326. https://10.1016/0741-8329(90)90090-y [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV and Spanagel R (2005). “Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases.” Alcohol Clin Exp Res 29(7): 1139–1145. https://10.1097/01.alc.0000171928.40418.46 [DOI] [PubMed] [Google Scholar]
- Sircar R and Sircar D (2005). “Adolescent rats exposed to repeated ethanol treatment show lingering behavioral impairments.” Alcohol Clin Exp Res 29(8): 1402–1410. [DOI] [PubMed] [Google Scholar]
- Skelly MJ, Chappell AE, Carter E and Weiner JL (2015). “Adolescent social isolation increases anxiety-like behavior and ethanol intake and impairs fear extinction in adulthood: Possible role of disrupted noradrenergic signaling.” Neuropharmacology 97: 149–159. https://10.1016/j.neuropharm.2015.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2000). “The adolescent brain and age-related behavioral manifestations.” Neuroscience and Biobehavioral Reviews 24: 417–463. [DOI] [PubMed] [Google Scholar]
- Strong MN, Yoneyama N, Fretwell AM, Snelling C, Tanchuck MA and Finn DA (2010). ““Binge” drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood.” Horm Behav 58(1): 82–90. https://10.1016/j.yhbeh.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambour S, Brown LL and Crabbe JC (2008). “Gender and age at drinking onset affect voluntary alcohol consumption but neither the alcohol deprivation effect nor the response to stress in mice.” Alcohol Clin Exp Res 32(12): 2100–2106. https://10.1111/j.1530-0277.2008.00798.x [DOI] [PubMed] [Google Scholar]
- White AM, Ghia AJ, Levin ED and Swartzwelder HS (2000). “Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol.” Alcohol Clin Exp Res 24(8): 1251–1256. [PubMed] [Google Scholar]
- Wolffgramm J (1990). “Free choice ethanol intake of laboratory rats under different social conditions.” Psychopharmacology (Berl) 101(2): 233–239. https://10.1007/BF02244132 [DOI] [PubMed] [Google Scholar]
- Wolstenholme JT, Mahmood T, Harris GM, Abbas S and Miles MF (2017). “Intermittent Ethanol during Adolescence Leads to Lasting Behavioral Changes in Adulthood and Alters Gene Expression and Histone Methylation in the PFC.” Front Mol Neurosci 10: 307 https://10.3389/fnmol.2017.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis RM, Wolstenholme JT, Bagdas D, Bettinger JC, Miles MF and Damaj MI (2019). “Adolescent but not adult ethanol binge drinking modulates ethanol behavioral effects in mice later in life.” Pharmacol Biochem Behav 184: 172740 https://10.1016/j.pbb.2019.172740 [DOI] [PMC free article] [PubMed] [Google Scholar]