3. Pipeline for validating MR imaging biomarkers for use in clinical practice or clinical trials and the status of different MR modalities in the pipeline for different degenerative ataxia categories.
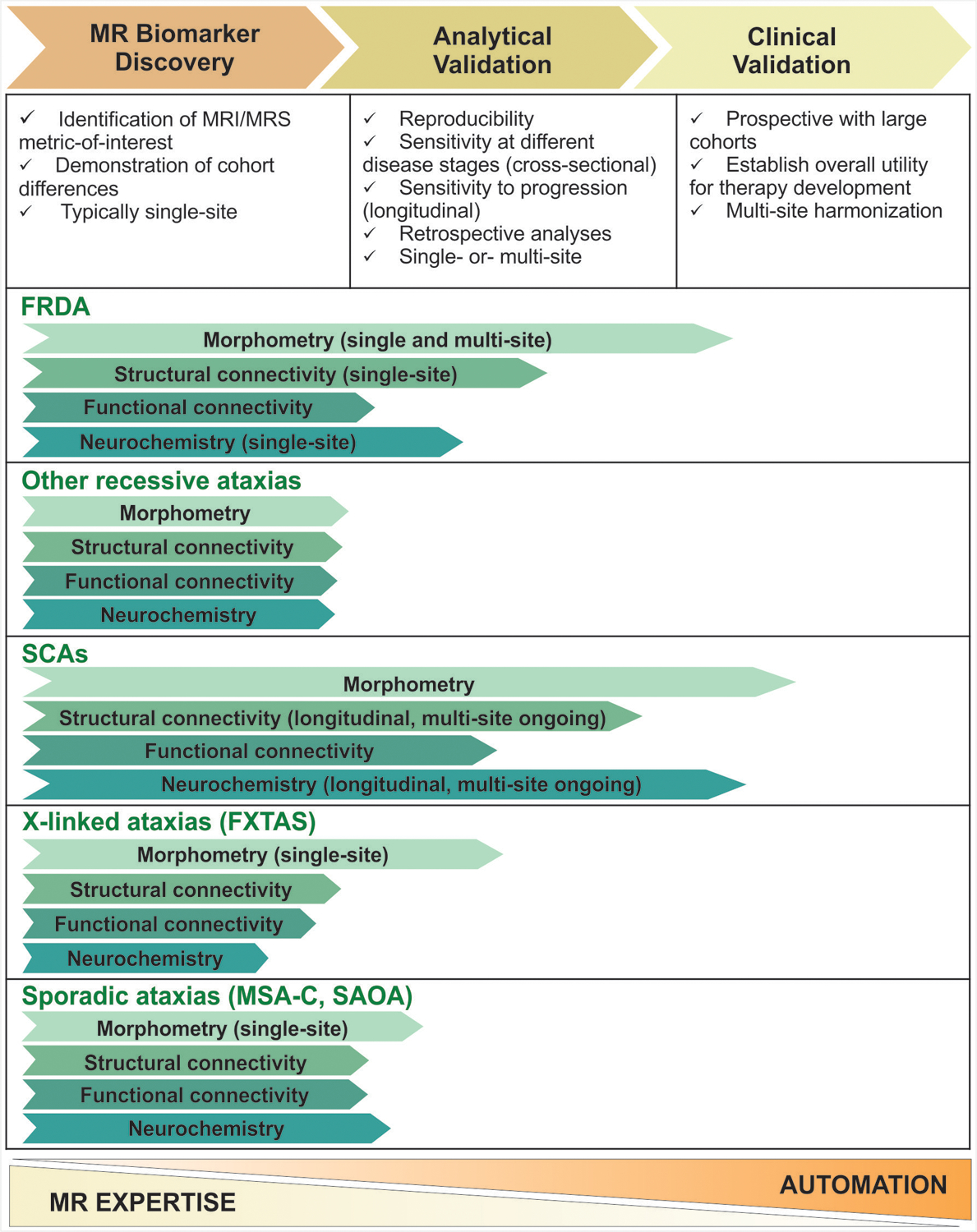
Note that the final phase, regulatory approval (“Qualification”) that follows the first three phases is not shown. The lengths of the arrows are meant to provide the reader an approximate idea of the place of the different markers in the pipeline relative to each other and relative to the phases defined on top. For example, prospective multi-site morphometry studies with large FRDA cohorts are in the planning stage, hence the arrow ends early in the third phase. Similarly, multi-site morphometry studies with relatively large SCA cohorts have been completed (e.g. EUROSCA), however sample sizes are still limited relative to natural history studies that have validated clinical scales. Typically, earlier stage biomarker discovery studies are conducted at sites with substantial MR expertise and as the identified biomarker moves through the pipeline, increased automation in data acquisition and analysis is necessary for clinical utility, as represented by the scale at the bottom.
