Scheme 1:
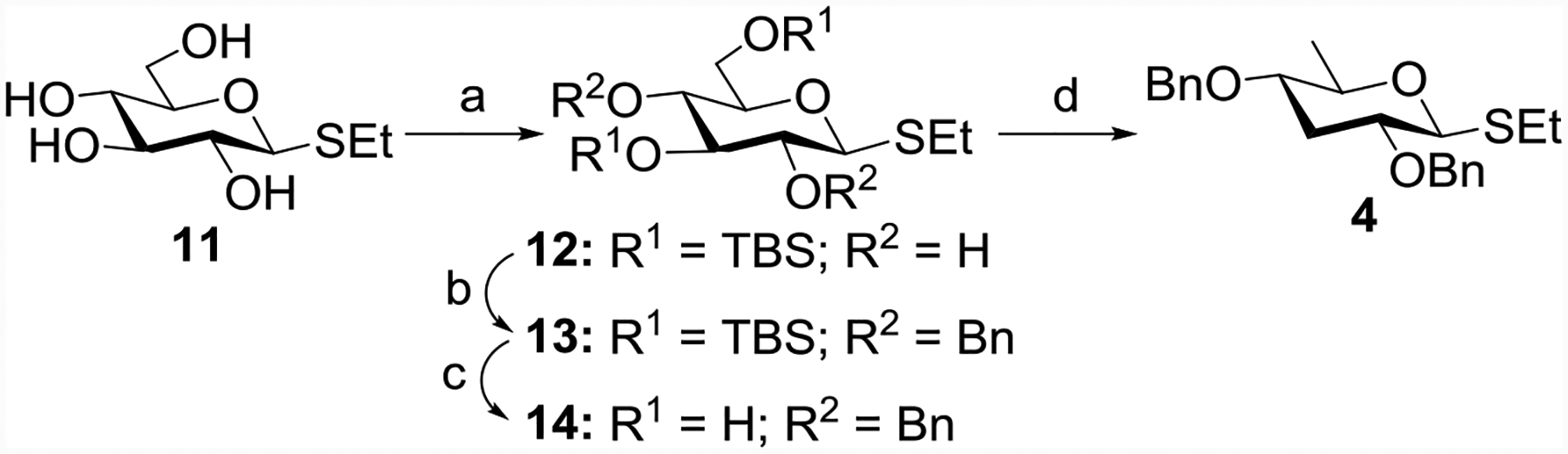
Reagents and conditions: (a) TBSCl, imidazole, DMF, r t, 12 h, 66%; (b) BnBr, NaH, DMF, 2 h, 0 °C, 88%; (c) p-TSA, CH3OH, r t, 2 h, 89%; (d) (i) NaH, imidazole, CS2, CH3I, THF, r t; (ii) tri-n-butyltin hydride (TBTH), azobis-isobutyronitrile (AIBN), reflux, 45% yield in one pot.
