Figure 2.
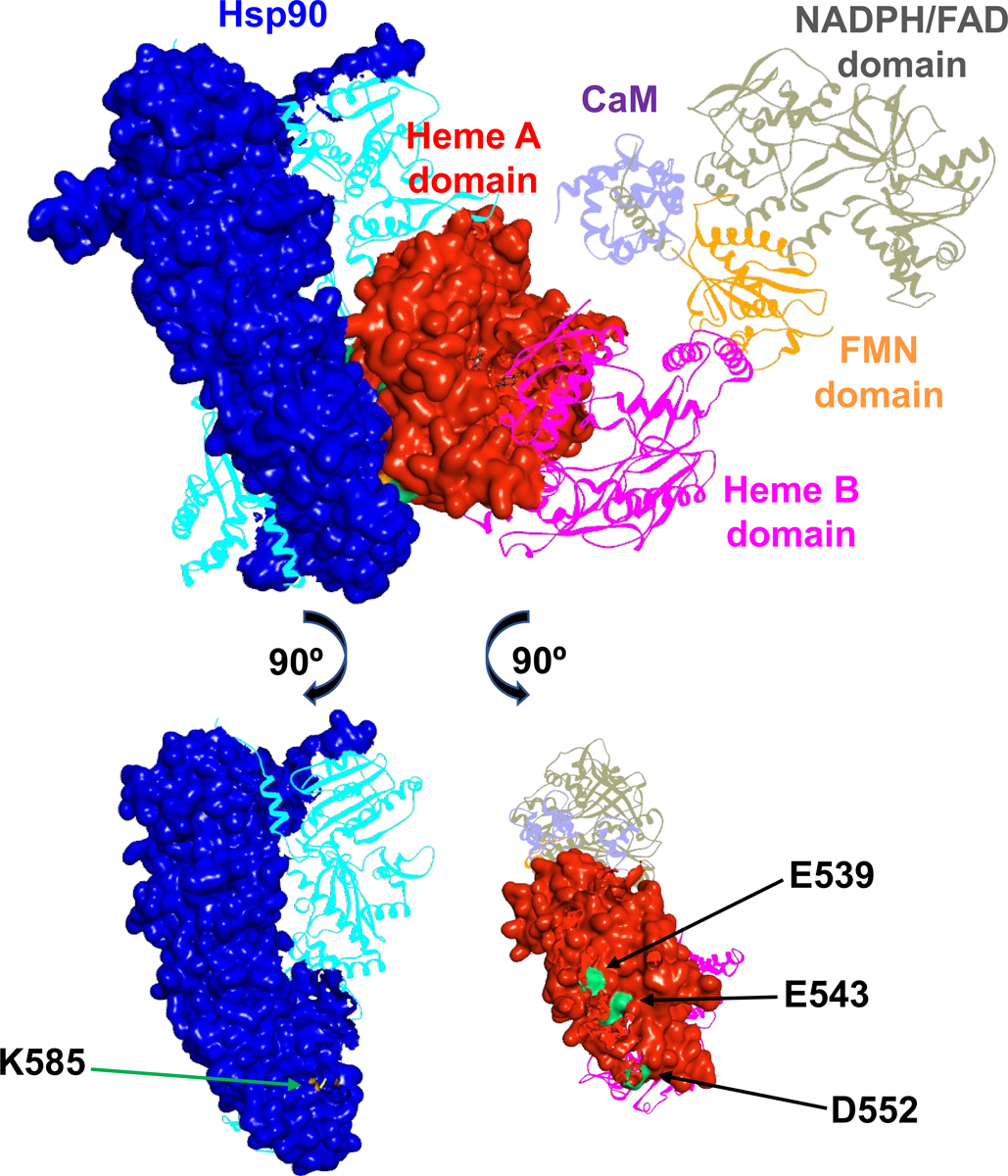
A docking model of rat nNOS holoprotein in complex with human Hsp90α in a closed conformation. The subunits in the dimeric Hsp90 protein are shown in blue and cyan, and the domains of nNOS bound with CaM (purple) are colored differently. Molecular surfaces of the interacting Hsp90 subunit and nNOS heme A domain are depicted, while the other modules are shown in ribbons. The FMN domain departs from the NADPH/FAD domain and is on its way to dock to the heme A domain. Only one reductase domain of nNOS is shown for clarity, along with the dimeric heme-containing oxygenase domain. The bottom panels illustrate the Hsp90 K585 residue (colored in orange) and the rat nNOS heme domain E539, E543 and D552 residues (colored in green) at the predicted Hsp90-nNOS interface.
