Summary
Plg-RKT, is a structurally unique transmembrane plasminogen receptor with both N- and C-terminal domains exposed on the extracellular face of the cell. Its C-terminal lysine functions to tether plasminogen to cell surfaces. Over expression of Plg-RKT increases cell surface plasminogen binding capacity while genetic deletion of Plg-RKT decreases plasminogen binding. Plasminogen binding to Plg-RKT results in promotion of plasminogen activation to the broad spectrum serine protease, plasmin. This function is promoted by the physical association of Plg-RKT with the urokinase receptor (uPAR). Plg-RKT is broadly expressed in cells and tissues throughout the organism and its sequence is remarkably conserved phylogenetically. Plg-RKT also is required for lactation and, thus, is necessary for survival of the species. This review provides an overview of established and emerging functions of Plg-RKT and highlights major roles for Plg-RKT in both the initiation and resolution of inflammation. While the roles for Plg-RKT in the inflammatory response are predominantly plasmin(ogen)-dependent, its role in lactation requires both plasminogen-dependent and plasminogen-independent mechanisms. Furthermore, the functions of Plg-RKT are dependent on sex. In view of the broad tissue distribution of Plg-RKT, its role in a broad array of physiological and pathological processes should provide a fruitful area for future investigation.
Keywords: Fibrin, Fibrinolysis, Plasminogen, PLG-R(KT) protein, Receptors
1. Introduction
This review article focusses on functions of the novel plasminogen receptor, Plg-RKT. Here we briefly summarize general concepts that apply to the interaction of plasminogen with cells, followed by a summary of unique aspects of Plg-RKT sequence and topology and its in vitro function. We then discuss, in depth, recent data implicating a major role of Plg-RKT in innate immune function as a plasminogen receptor as well as surprising data revealing an unexpected role of Plg-RKT in lactation in which Plg-RKT exhibits both plasminogen-dependent and plasminogen-independent roles. We discuss how sex as a biological variable affects Plg-RKT function. Finally, we summarize emerging functional roles of Plg-RKT in pathophysiological processes.
2. Key Features of the Interaction of Plasminogen with Cells
2.1. Mechanisms for promoting association of plasmin activity with cell surfaces
The ability of plasminogen to specifically bind to cell surfaces was first demonstrated on platelets [1]. Subsequent studies have shown that plasminogen binding sites are present almost ubiquitously on eukaryotic cells and in tissues as well as on many prokaryotic cells (reviewed in [2]). Localization of plasminogen on cell surfaces promotes its activation to the broad spectrum serine protease, plasmin, in a reaction mechanism that is promoted when plasminogen activators [tissue plasminogen activator (t-PA) or urokinase (u-PA)] are colocalized with plasminogen (reviewed in [3]), analogous to plasminogen activation on fibrin to promote fibrinolysis [4]. In addition, plasminogen activation is promoted when the interaction of Glu-plasminogen (the native, circulating form of plasminogen) with cells induces a conformation exposing the cleavage site for plasmic removal of its N-terminal 77 amino acids [5] to form the more readily activatable Lys-plasminogen [6–8]. As an additional mechanistic consideration, enhancement of plasminogen activation on eukaryotic cell surfaces is lost following treatment of cells with carboxypeptidase B (CpB) [9]. CpB proteolytically removes C-terminal basic residues. Hence, plasminogen binding proteins exposing C-terminal basic residues on cell surfaces are predominantly responsible for stimulation of plasminogen activation. Furthermore, plasmin formed on the cell surface is retained on the cell membrane and is protected from inactivation by its major inhibitor, α2-antiplasmin [10, 11], most likely due to competition between cell surface C-terminal basic residues with the C-terminal lysine of α2-antiplasmin that is required for the initial interaction with plasmin [12].
2.2. Physiological and pathophysiological roles of cell-associated plasmin
Localization of the broad spectrum proteolytic activity of plasmin on cells has important consequences for physiological and pathological processes, many of which require fibrin proteolysis as well as degradation of extracellular matrix (ECM) components to allow cells to migrate, including the inflammatory response, wound healing, oncogenesis, metastasis, myogenesis and muscle regeneration (reviewed in [3]). Prohormone processing, neurite outgrowth and fibrinolysis are also regulated by cell-associated plasmin (reviewed in [3]). Cell-bound plasmin also stimulates cell signaling pathways to induce release of cytokines, reactive oxygen species and other inflammatory mediators [13, 14].
2.3. Cell surface plasminogen binding proteins
The diversity of cell types that bind plasminogen as well as the high capacity of cells for plasminogen (e.g. [1, 15]) has led to identification of numerous plasminogen receptors. Prior to the discovery of Plg-RKT, CpB-sensitive cellular plasminogen receptors comprised two groups: 1) proteins synthesized with C-terminal basic residues and having well recognized intracellular functions, including α-enolase [16, 17], cytokeratin 8 [18, 19], S100A10 (in complex with annexin A2 within the annexin A2 heterotetramer)[20], TIP49a [21] and histone H2B [22] and; 2) proteins that undergo proteolytic processing in order to expose a C-terminal basic residue to permit plasminogen binding, including actin [23–25]. There is also a CpB-insensitive component of plasminogen binding to eukaryotic cells that does not promote plasminogen activation [9], which includes tissue factor [26] and non-proteinaceous gangliosides [27]. Several transmembrane proteins (αIIbβ3 [28], αMβ2 [29, 30] and α5β1 [30], as well as amphoterin [31] and GP330 [32]) bind plasminogen but are not synthesized with C-terminal basic residue and it has not been established whether these proteins undergo proteolysis to reveal C-terminal basic residues. Detailed reviews on the identification, functions and mechanisms of cell surface-association of the plasminogen receptors discussed above can be found in the volume introduced by reference [33].
3. Discovery of Plg-RKT
3.1. Proteomic discovery of the novel plasminogen receptor, Plg-RKT
In the absence of identification of an integral membrane protein exposing a carboxyl terminal basic residue on the cell surface, a proteomic approach was applied as a sensitive technique that could potentially reveal such a protein. Cell surface labeling of an inducible monocytoid stem cell line, followed by targeted proteolysis with CpB, affinity chromatography on plasminogen and streptavidin and multidimensional protein identification technology (MudPIT) was performed [3, 34]. One protein with a predicted transmembrane sequence and a C-terminal basic residue was identified: the hypothetical protein, C9orf46 homolog (IPI00136293), homologous to the protein predicted to be encoded by human chromosome 9, open reading frame 46 [34]. Interestingly, the MudPIT analysis also detected peptides from other proteins previously characterized as plasminogen receptors on monocytes: α-enolase, histone H2B, gamma actin, S100A10, annexin A2 and β2 integrin, as validation of the method.
3.2. Characteristics of Plg-RKT
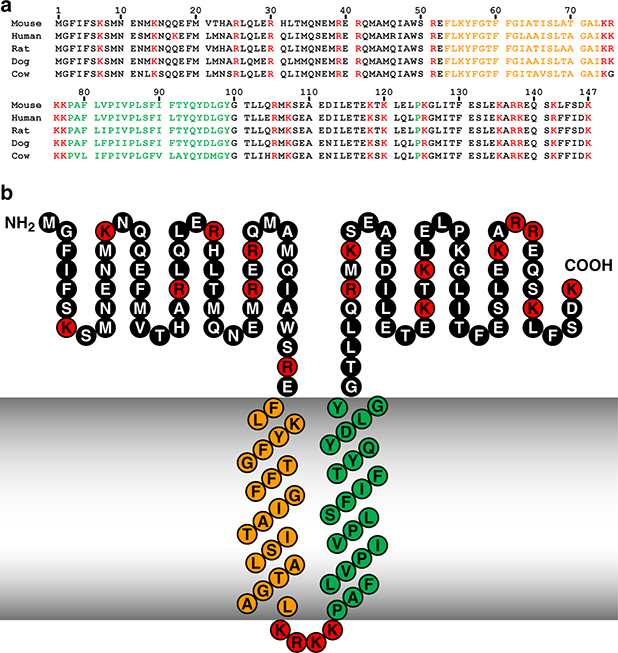
The C9orf46 homolog murine DNA sequence encodes a protein of 147 amino acids with a predicted molecular mass of 17,261 Da and a C-terminal lysine (Fig. 1A). The protein was named Plg-RKT, to indicate a plasminogen receptor with a C-terminal lysine and a transmembrane domain. The sequences of over sixty-five mammalian orthologs are now available and alignment of these sequences shows high identity (e.g. human vs. chimpanzee = 99% identity; human vs. grizzly bear = 87.8% identity) with no gaps in the sequences. Importantly, a C-terminal lysine is encoded for all sequenced mammalian orthologs (e.g. [35]). Furthermore, DNA sequences of phylogenetically lower species (green lizard, xenopus, and zebrafish) encode C-terminal lysines. Also contained within the Plg-RKT sequence is a conserved DUF2368 domain (spanning amino acids 1–135), an uncharacterized protein with unknown function that is evolutionarily conserved from nematodes to humans. The Plg-RKT orthologs of lower organisms (e.g. Drosophila, Paramecium and the sea urchin, Strongylocentrotus purpuratus) predict proteins of different lengths and do not always predict C-terminal lysines. Notably, plasminogen is considered to have evolved evolutionarily with protochordates [36]. Thus, organisms evolutionarily below protochordates might not need the C-terminal lysine of Plg-RKT.
Figure 1. Alignment of predicted amino acid sequences of mouse, human, rat, dog and cow orthologs of Plg-RKT (A) and the structural model of Plg-RKT (B) show high interspecies sequence homology.
Green indicates amino acids within the predicted primary transmembrane helix. Orange indicates amino acids within the predicted secondary transmembrane helix. Red indicates basic amino acids. This research was originally published in Blood, Andronicos, N.M., Chen, E.I., Baik, N., Bai, H., Parmer, C.M., Kiosses, W.B., Kamps, M.P., Yates, J.R., III, Parmer, R.J., Miles, L.A., Proteomics-based discovery of a novel, structurally unique, and developmentally regulated plasminogen receptor, Plg-RKT, a major regulator of cell surface plasminogen activation, Blood. 2010, 115: 1319–30. © the American Society of Hematology.
Predictive websites indicate a preferred topology model with two transmembrane helices extending from F53-L73 (oriented from outside the cell to inside the cell) and P78-Y99 (oriented from inside the cell to outside the cell) with a short 4 basic amino acid cytoplasmic sequence (Fig. 1B) [34]. Thus, a 52 amino acid N-terminal region and a 48 amino acid C-terminal tail with a C-terminal lysine are predicted to be exposed on the cell surface. This model is supported by several lines of evidence: 1) In phase separation experiments Plg-RKT partitions to the detergent phase, consistent with integral membrane protein behavior [34, 37]; 2) Protease accessibility experiments support extracellular exposure of both N-and C-termini of Plg-RKT [35, 38]; 3) Peptides corresponding to Plg-RKT are not recovered when intact cells are treated with CpB, prior to proteomic analysis, in accordance with cell surface exposure of the C-terminus of Plg-RKT [34]; and 4) A mAB raised against the C-terminal peptide of Plg-RKT reacts with the cell surface [34, 37, 39].
Plg-RKT exhibits key requisite attributes of a plasminogen receptor. Plasminogen binds directly to the C-terminal peptide of Plg-RKT and a mAB raised against this region reacts with plasminogen binding sites on cells [34]. Overexpression of Plg-RKT increases cell surface plasminogen binding capacity [37]. Genetic deletion of Plg-RKT markedly decreases plasminogen binding to cells [39]. Plg-RKT promotes plasminogen activation by both t-PA [34] and u-PA [40]. Plg-RKT colocalizes [34, 39] and co-immunoprecipitates [37] with uPAR as a mechanism to promote plasminogen activation. Notably, t-PA binds specifically to a peptide corresponding to the C-terminus of Plg-RKT [34]. Despite the potential to compete for binding to Plg-RKT, the relative concentrations of circulating plasminogen and t-PA should permit simultaneous binding of both ligands to distinct Plg-RKT molecules so that each t-PA molecule should be bound proximally to several plasminogen molecules to promote plasminogen activation by t-PA [41].
Plg-RKT has a broad tissue distribution, as determined by probing human and mouse tissue microarrays with a panspecific monoclonal antibody [40] raised against a synthetic nonapeptide corresponding to the C-terminus of rat Plg-RKT. Plg-RKT is expressed in murine and human tissues including neuroendocrine tissue (thyroid and adrenal gland), brain, spleen, lymph nodes, vasculature, and very prominently, in epithelial tissues including kidney, breast, lung, intestine and colon [42]. In mammary tissue, high Plg-RKT expression is present in epithelial tissue of tubuloalveolar glands and lactiferous ducts [42, 43]. In lungs, Plg-RKT expression is notable on pseudostratified and simple columnar epithelium in bronchi. And absorptive epithelium in small intestine and colon exhibit high Plg-RKT expression [42]. By Western blotting, high expression of Plg-RKT is detected in human lymphocytes, peripheral blood monocytes, and endothelial cells, with much lower expression in neutrophils, while not detectable in RBC [40]. The broad distribution in tissues that are exposed to plasminogen suggests that Plg-RKT may regulate proteolytic activity throughout the organism.
4. Role of Plg-RKT in Innate Immunity
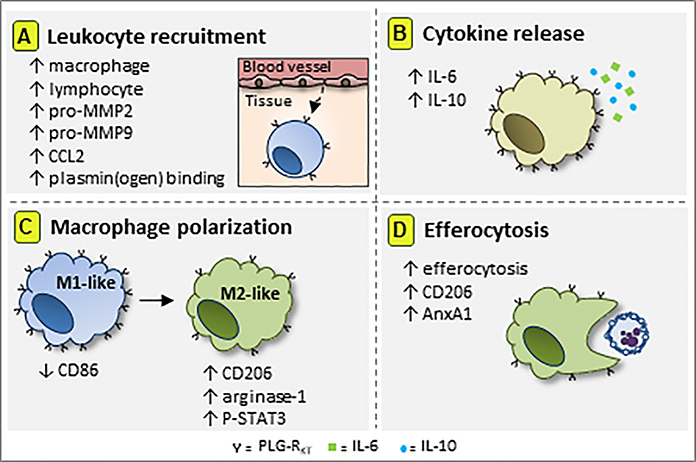
In response to infectious or sterile injurious stimuli, an inflammatory response is initiated leading to increased vascular permeability, release of pro-inflammatory mediators and subsequent leukocyte recruitment [44]. Subsequently, anti-inflammatory mechanisms come into play, followed by the resolution of inflammation, an active process that functions to restore tissue homeostasis [45]. Plg-RKT exerts a major impact on each of these processes as discussed below and as summarized in Figure 2.
Figure 2. Schematic representation of the role of Plg-RKT in innate immunity.
Plg-RKT receptor is required for macrophage and lymphocyte recruitment to inflammatory sites. (A) Mechanistically, this effect is mediated by plasmin(ogen) binding and activation via Plg-RKT, by the release of the monocyte chemoattractant CCL2 and by activation of the matrix metalloproteinases pro-MMP2 and pro-MMP-9. (B) Plg-RKT receptor increases the release of IL-6 and IL-10 cytokines. (C) Plg-RKT is preferentially expressed on M1-like macrophages, while during inflammation Plg-RKT decreases CD86 expression. In a similar way, Plg-RKT promotes expression of macrophage M2 markers and (D) enhances efferocytosis of apoptotic cells (4). This diagram has not been published previously.
4.1. Regulation of leukocyte recruitment by Plg-RKT
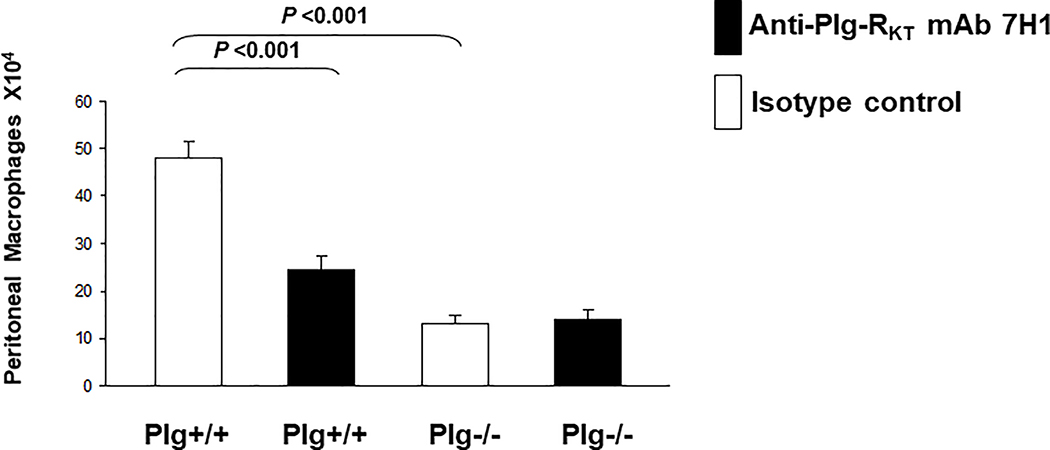
Studies in plasminogen-deficient mice have revealed a key role for plasminogen in macrophage and lymphocyte recruitment during the inflammatory response [46–49]. Previous studies indicate that active plasmin is required to promote leukocyte recruitment [49] and plasminogen-dependent macrophage recruitment is mediated by CpB-sensitive plasminogen binding sites [50]. Consistent with these requirements, injection of mice with anti-Plg-RKT mAB impairs mononuclear leukocyte recruitment in a model of sterile peritonitis induced by injection of thioglycollate. (Treatment with thioglycollate results in sequential recruitment of neutrophils, monocytes and lymphocytes to the peritoneum.) Treatment with anti-Plg-RKT mAB inhibits both macrophage recruitment (by 53%) and lymphocyte recruitment (by 60%) without affecting either neutrophil or eosinophil recruitment [40]. Macrophage and lymphocyte recruitment are also impaired in this model in plasminogen deficient mice, without an effect on neutrophils [49, 51]. Similar effects on selective leukocyte recruitment occur in response to pleural injection of plasmin [52, 53] or LPS [54]. The absence of a genotype effect on neutrophil recruitment is consistent with relatively low expression of Plg-RKT on neutrophils [40] and relatively low plasminogen binding capacity of neutrophils [55]. When anti-Plg-RKT mAB is injected into plasminogen deficient mice, there is no further decrease in macrophage recruitment, illustrating that Plg-RKT functions to bind plasmin(ogen) in the thioglycollate model [40] (Fig. 3).
Figure 3. Effect of Plg-RKT on thioglycollate-induced monocyte recruitment.
Both plasminogen deficient (Plg−/−) and wild type littermates (Plg+/+) mice were injected intravenously with either mAb 7H1 (■) or isotype control (□) (500μg). After 30 minutes thioglycollate was injected intraperitoneally. A second injection of antibody was given 24 hours later. After 72 hours, thioglycollate-recruited cells were collected by peritoneal lavage and macrophages were purified by adherence. The adherent cells were detached and counted using a hemocytometer. Data represent mean ± S.E.M. n=5/group). This research was originally published in Blood, Lighvani, S., Baik, N., Diggs, J.E., Khaldoyanidi, S., Parmer, R.J, and Miles, L.A. Regulation of macrophage migration by a novel plasminogen receptor Plg-RKT. Blood, 2011, 118:5622–5630. © the American Society of Hematology.
Effects of genetic deletion of Plg-RKT on macrophage recruitment have been demonstrated in two models of inflammation. In time course experiments there is no effect of Plg-RKT deletion until 72 hr following thioglycollate injection when total leukocyte recruitment is significantly impaired and macrophage recruitment is 76% lower in Plg-RKT−/− mice, compared with Plg-RKT+/+ littermate controls [56]. In the second model, stimulation of inflammation by intrapleural injection of LPS results in time-dependent recruitment of leukocytes into the pleural cavity with early neutrophilic recruitment and resolution at 48 hr when neutrophils are scarce and mononuclear cell infiltration is maximal [57, 58]. In this model of pleurisy, recruitment of mononuclear cells and pleural levels of monocyte chemoattractant chemokine, CCL2, are significantly lower in Plg-RKT−/− mice compared to Plg-RKT+/+ littermates, with no significant effect of genotype on recruitment of neutrophils or levels of the neutrophil chemoattractant chemokine, CXCL-1 [54]. A similar effect is observed in plasminogen deficient mice [54], suggesting plasminogen dependence of the functions of Plg-RKT in this model.
4.1.1. Mechanisms by which Plg-RKT regulates leukocyte recruitment
The in vivo studies summarized above are consistent with Plg-RKT functioning to bind plasminogen and promote plasminogen activation to plasmin on the surfaces of migrating monocytoid cells and lymphocytes. In vitro studies also support the plasminogen binding functions of Plg-RKT. Anti-Plg-RKT mAB blocks plasminogen dependent monocytoid cell migration through Matrigel as well as plasminogen-dependent chemotactic migration in response to the monocyte chemoattractant, CCl2 (MCP-1) [40].
Plg-RKT plays a role in the activation of pro-MMPs. In the thioglycollate-induced sterile peritonitis model, activation of pro-MMP-9 is required for plasmin(ogen)-dependent macrophage recruitment [49] and activation of pro- MMP-9 and pro-MMP-2 is observed in peritoneal fluid [40]. Injection of anti-Plg-RKT mAB decreases pro-MMP-9 activation by 64% and pro-MMP-2 activation by 44% [40]. In studies in vitro, anti-Plg-RKT mAB blocks activation of pro-MMP-9 and pro-MMP-2 synthesized by monocytoid cells [40].
It has recently been recognized that Plg-RKT may additionally regulate CCL2 levels as a new mechanism for regulation of macrophage migration. In the LPS-induced pleurisy model the lower number of mononuclear cells recruited to the pleural cavity is accompanied by a significantly lower level of CCL2 in the pleural cavity of Plg-RKT−/− mice, compared with Plg-RKT+/+ littermates [54]. This is also observed in plasminogen deficient mice [54]. Similarly, peritoneal CCL2 levels are decreased in plasminogen deficient mice following biomaterial implantation [48]. Furthermore, treatment of mice with plasmin(ogen) increases monocyte migration to the pleural cavity via a mechanism dependent on CCL2/CCR2 [52]. Strikingly, there is no effect of specific deletion of Plg-RKT in myeloid cells (mPlg-RKT−/− mice) on recruitment to the pleural cavity or on pleural CCL2 levels [54]. Thus, Plg-RKT deletion in other cell types may affect synthesis of CCL2 and impact monocyte recruitment in mice with global deletion of Plg-RKT.
Preferential expression of Plg-RKT on pro-inflammatory monocyte and macrophages is a newly recognized mechanism for regulation of their migration and recruitment. Pro-inflammatory human intermediate monocytes (CD14++CD16+) express significantly higher levels of Plg-RKT on their surfaces compared with classical monocytes (CD14++CD16-) and nonclassical monocytes (CD14+CD16++) and this is paralleled by significantly higher plasminogen binding capacity on intermediate monocytes [39]. Mechanistically, intermediate monocytes exhibit plasmin(ogen)-dependent cell migration that is blocked by anti-Plg-RKT mAB [39]. Furthermore, in murine blood, the pro-inflammatory Ly6Chigh monocyte subset expresses significantly more Plg-RKT and plasminogen binding capacity than the Ly6Clow subset and both wild type monocyte subsets bind significantly more plasminogen than Plg-RKT−/− subsets [39]. In the sterile peritonitis model Ly6Chigh monocyte recruitment is decreased by 57% in Plg-RKT−/− mice compared to Plg-RKT+/+ littermates, but there is no genotype effect on recruitment of Ly6Clow cells [39].
4.2. Role of Plg-RKT in inflammatory cytokine synthesis and release
The ability of plasmin to stimulate intracellular signaling pathways and cytokine release by monocytes and macrophages is well established in vitro and requires an interaction of plasmin(ogen) with the cell surface [14, 59–61]. Following thioglycollate stimulation, peritoneal levels of IL-6 are increased over 7-fold in Plg-RKT+/+ mice, but less than 2-fold in Plg-RKT−/− mice and IL-10 levels are 3 fold lower in Plg-RKT−/− compared with Plg-RKT+/+ mice [39]. (IL-10 has anti-inflammatory properties and is also induced under inflammatory conditions [62, 63]).
4.3. Regulation of resolution of inflammation by Plg-RKT
4.3.1. Regulation of macrophage polarization by Plg-RKT
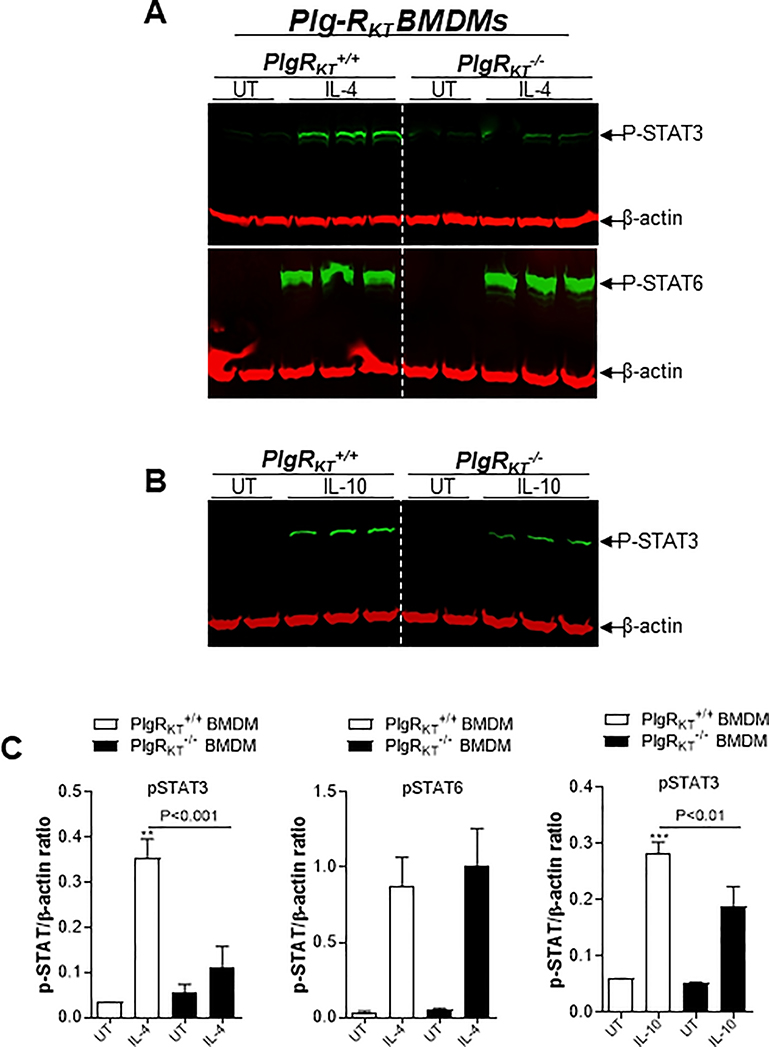
During the resolution phase of inflammation, macrophages are reprogrammed to a spectrum of M2-like resolving phenotypes [64]. In the LPS-induced pleurisy model, a higher proportion of M1-like macrophages are present in the pleural exudates of both Plg-RKT−/− and plasminogen deficient mice, compared with their wild type littermates, suggesting an impairment in polarization to the M2-like phenotype, with reduced expression of the engulfment and recognition molecules, CD206 and Annexin A1 [54]. Furthermore, polarization of Plg-RKT−/− and plasminogen deficient bone marrow derived macrophages (BMDMs) toward an M2-like phenotype (with either IL-4 or IL-10) is impaired, with reduction in the expression of well established M2 markers resulting from IL-4 stimulation (CD206 and arginase-1) and IL-10 polarizing agents (TGFβ) [54]. On the other hand, M1-like Plg-RKT−/− BMDMs exhibit increased expression of the M1 marker, CD86 [54]. IL-4 can stimulate macrophage polarization through either the STAT6 (canonical) or STAT3 (non-canonical) pathways, while IL-10 triggers the STAT3 pathway [65]. Treatment of both Plg-RKT−/− and plasminogen deficient BMDMs with IL-4 or IL-10 results in a reduction in phosphorylated STAT3 but not phosphorylated STAT6 [54] (Fig. 4). This suggests a mechanism in which plasmin(ogen) interacts with Plg-RKT to stimulate STAT3 phosphorylation and is the first demonstration of intracellular signalling mediated by this receptor.
Figure 4. Effect of plasmin(ogen) treatment and deletion of Plg-RKT on STAT signaling pathways.
Bone marrow derived macrophages (BMDMs) from Plg-RKT+/+ and Plg-RKT−/− mice were washed 3 times with serum free media and then treated with either IL-4 (20ng/mL), LPS (10ng/mL) + IFN (10ng/mL), IL-10 (20ng/mL) or untreated (UT) for 30 minutes (A,B). Cell lysates were analysed by Western blotting for the indicated antigens. β-actin was used as a loading control. Densitometry analyses are shown (C). This research was originally published in Vago, J.P., Sugimoto, M.A., Lima, K.M., Lima, G.L., Baik, N., Teixeira, M.M., Perretti, M., Parmer, R.J., Miles, L.A. and Sousa, L.P. Plasminogen and the plasminogen receptor, Plg-RKT, regulate macrophage phenotypic and functional changes. Front. Immunol. 28 June 2019, 10:1458.
Interestingly, proinflammatory M1-like macrophages exhibit significantly more cell surface Plg-RKT than either unpolarized or M2-like macrophages [39]. Thus, Plg-RKT functions to promote polarization and is then down-regulated as a potential negative feedback mechanism.
4.3.2. Regulation of Efferocytosis by Plg-RKT
Following their recruitment to inflammatory sites, and having exerted effector functions, neutrophils undergo apoptosis. An essential step to decrease tissue exposure to toxic contents of dying cells is their phagocytic engulfment by macrophages (efferocytosis) [66]. Plasmin(ogen) plays a key role in phagocytosis and this requires its interaction with cell surfaces and protein synthesis [53, 67–70]. Efferocytosis in vitro is also significantly decreased in Plg-RKT−/− and plasminogen deficient BMDMs [54]. When macrophage recruitment is stimulated by intraperitoneal injection of zymosan followed by injection of fluorescently labelled apoptotic neutrophils, engulfment of neutrophils is significantly decreased in Plg-RKT−/−, mPlg-RKT−/− and plasminogen deficient mice relative to their wild type littermate controls [54]. Macrophage expression of the engulfment and recognition molecules, CD206 and AnxA1 is significantly lower in these deficient mice [54]. Indeed, the expression of AnxA1, among other sets of genes involved in phagocytosis, is modulated in liver following injection of anti-RBC autoantibody and in spleen in response to injection of apoptotic thymocytes in plasminogen deficient mice [68] and plasmin(ogen) promotes efferocytosis in an AnxA1-dependent fashion [58]. Thus, binding of plasmin(ogen) to Plg-RKT may promote a mechanism to stimulate AnxA1 expression to further amplify the process of efferocytosis.
5. Sex as a Biological Variable in the Plasminogen Activation System
Sex has significant effects on the plasminogen activation system. Circulating plasminogen levels in male mice are significantly lower (46%) than female mice [56]. In humans, female sex is associated with higher plasminogen levels [71]. Female murine monocytes express significantly more cell surface Plg-RKT than male monocytes [39]. And the number of monocytes recruited to the peritoneum in the thioglycollate model is markedly greater in female compared with male mice [39]. Thus, females may have a more efficient activation of plasminogen on cell surfaces, which may significantly affect outcomes of many pathological states.
6. Role of Plg-RKT in Lactation
Plg-RKT deficient mice are viable and fertile and offspring of heterozygous matings are born according to the expected Mendelian ratios and there is no effect of genotype on survival [56]. However, Plg-RKT−/− females (on the C57Bl/6J background) exhibit a severe lactation defect, resulting in death of all offspring within two days after birth (Fig. 5) [43]. Pups of Plg-RKT+/− female mice have survival rates intermediate between offspring of Plg-RKT−/− and Plg-RKT+/+ females, indicating a significant maternal gene dosage effect [56]. The effect of maternal genotype on pup survival is not strain-dependent as the lactational defect is also present in Plg-RKT deficient mice on the 129XS/SVJ background [43]. Pup death is consistent with the inability of Plg-RKT−/− mothers to successfully lactate as milk is not present in the stomachs of offspring of Plg-RKT−/− mothers. In addition, cross fostering demonstrates that the lactation defect is with Plg-RKT−/− mothers [43]. This section reviews evidence for mechanisms by which Plg-RKT regulates lactation.
Figure 5. Plg-RKT null mice cannot successfully lactate.
Survival data are shown for a cohort of offspring of Plg-RKT+/+ (dashed lines) and Plg-RKT−/− (solid lines) primiparous female littermates: 48 offspring of Plg-RKT+/+ mice and 30 offspring of Plg-RKT−/− mice. **** P<0.0001 Log-rank (Mantel Cox) test]. This research was originally published in Miles, L.A., Baik, N., Bai, H., Makarenkova, H.P., Kiosses, W.B., Krajewski, S., Castellino, F.J., Valenzuela, A., Varki, N.M., Mueller, B.M., Parmer, R.J. The plasminogen receptor, Plg-RKT, is essential for mammary lobuloalveolar development and lactation. J Thromb Haemost. 2018, 16(5):919–932. and is reprinted with permission from John Wiley and Sons.
6.1. Plg-RKT regulates milk production and ductal histology
Mammary gland masses of Plg-RKT−/− mice are significantly (50%) less than those of Plg-RKT+/+ mice. Furthermore, milk production is severely and significantly decreased in mammary glands of Plg-RKT−/− mothers, while there are no genotype-dependent effects on tissue prolactin levels in mammary glands [43]. The alveoli of Plg-RKT−/− glands are strikingly atrophic and disordered and ducts are distended. Amorphous eosinophilic material, suggesting high protein content, is present in alveoli and ducts of Plg-RKT−/− glands [43].
6.2. Plg-RKT regulates the composition of the ECM in lactating mammary glands
Lactogenesis requires the appropriate extracellular environment [72–74] and deletion of Plg-RKT results in marked dysregulation of the mammary ECM. Excessive collagen deposition is present within mammary adipose tissue surrounding the alveoli of the Plg-RKT−/− mammary glands and fibrosis is also present within the surrounding adipose tissue of Plg-RKT−/− mammary glands [43]. Consistent with the fibrotic ECM, cellular infiltrates are present within fibrotic areas of Plg-RKT−/− glands, with marked accumulation of macrophages [43].
Excessive fibrin deposition is present in the fibrotic mammary adipose tissue surrounding the alveoli and is also present within the distended alveoli and ducts of Plg-RKT−/− glands [43]. There are no genotype dependent effects on glandular levels of plasminogen activators, suggesting that the absence of Plg-RKT results in defective fibrinolysis due to impaired plasminogen activation.
6.3. Plg-RKT deletion affects fibrosis gene expression signatures in lactating mammary glands
In fibrosis gene transcription profiling of 84 genes in mammary glands harvested 2 days postpartum, 25 genes were identified for which expression in Plg-RKT−/− glands was significantly (P < 0.05) different than Plg-RKT+/+ glands by a factor of >2-fold [43]. Upregulation of remodeling enzymes and inhibitors (Mmp2, Mmp9 and Timp2), inflammatory cytokines and chemokines (Il1a, Il4 and TNF) and integrins (α2, β1, β3, β6, and β8) within the mammary tissue was consistent with the marked infiltration of macrophages observed in Plg-RKT−/− glands. In addition, TGFβ3 and its targets, Smad 2 and CTGF (connective tissue growth factor or CCN2) were strikingly upregulated. In addition, Mmp1a was markedly downregulated while Timp2 was markedly upregulated in Plg-RKT−/− glands, consistent with extensive collagen deposition in this genotype.
6.4. Plg-RKT deletion decreases epithelial cell proliferation and increases epithelial cell apoptosis
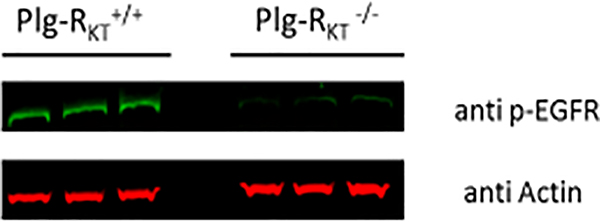
EGF was the most extensively down-regulated gene identified in Plg-RKT−/− glands (12-fold-less expression). Of ligands for the EGF receptor (EGFR), EGF is exclusively induced at lactation and is the most abundant of all differentially expressed cytokines and growth factors in luminal epithelial cells of lactating glands [75]. Correspondingly, levels of phospho-EGFR were markedly decreased in Plg-RKT−/− glands (Fig. 6). Furthermore, proliferating epithelial cells are abundant in Plg-RKT+/+ glands while not detectable in Plg-RKT−/− glands [43] (Fig. 7A).
Figure 6. Phosphorylated EGFR content is decreased in Plg-RKT−/− mammary glands.
Thoracic mammary glands were harvested from Plg-RKT+/+ and Plg-RKT−/− female mice two days following parturition. Glands were lysed and electrophoresed on 4–12% gradient gels under reducing conditions and western blotted with anti-phosphorylated EGFR. Ratios of pEGFR:Actin were significantly less in Plg-RKT−/− glands, (Plg-RKT−/− = 0.34 ±0.1; Plg-RKT+/+ = 0.90 ±0.01, P = 0.011, n=3). There were no genotype effects on EGFR levels. These data have not been published previously.
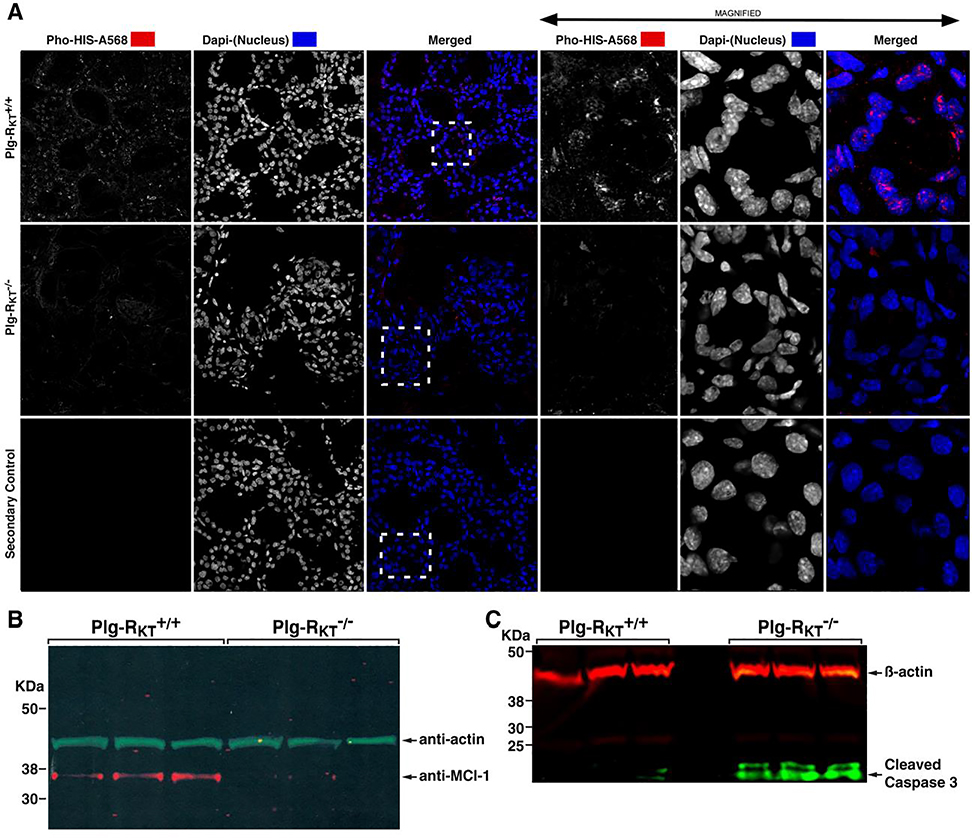
Figure 7.
Plg-RKT deletion decreases epithelial cell proliferation and increases epithelial cell apoptosis. Mammary glands were harvested two days postpartum from Plg-RKT+/+ and Plg-RKT−/− female mice. Frozen sections of abdominal mammary glands were immunostained with antibodies against Ser28-phosphorylated histone H3 (Pho-HIS-A568) and Dapi (shown in white for greater clarity). Boxes defined by dashed lines are the areas enlarged in right panels. Confocal images were captured using a Zeiss 710 Laser Scanning Confocal Microscope running the Zen 2016 software. 8 bit optical image section slices (sampled at 0.3μm intervals) were collected as 1024×1024 images, converted to maximum intensity projections for 2D analysis then imported into: Image Pro Premier, Image J and LSM Examiner software for further processing and quantitative analysis. (A). Thoracic glands were lysed and electrophoresed on 4–12% gradient gels under reducing conditions and western blotted with anti-Mcl-1 and anti-βactin (B) or anti-active (cleaved) caspase 3 and anti-βactin (C). This research was originally published in Miles, L.A., Baik, N., Bai, H., Makarenkova, H.P., Kiosses, W.B., Krajewski, S., Castellino, F.J., Valenzuela, A., Varki, N.M., Mueller, B.M., Parmer, R.J. The plasminogen receptor, Plg-RKT, is essential for mammary lobuloalveolar development and lactation. J Thromb Haemost. 2018, 16(5):919–932. and is reprinted with permission from John Wiley and Sons.
EGF orchestrates increased translation of the pro-survival Bcl-2 family protein, Mcl-1, and genetic deletion of Mcl-1 results in increased epithelial apoptosis [75]. Mcl-1 expression is observed in Plg-RKT+/+ glands, but is not detectable in Plg-RKT−/− glands (Fig. 7B) and, correspondingly, cleaved (activated) caspase 3 is present in Plg-RKT−/− glands, but not detectable in Plg-RKT+/+ glands [43] (Fig. 7C).
6.5. Interplay between plasminogen-dependent and plasminogen-independent functions in lactational development
A role for Plg-RKT in regulating fibrinolysis within mammary glands is supported by the excessive fibrin deposition in Plg-RKT−/− glands. Notably fibrin deposition is also observed in plasminogen deficient glands at postpartum day 2 [76]. The lactation defect in plasminogen deficient mice is rescued on a fibrinogen heterozygous background [76]. In contrast, fibrinogen heterozygosity does not rescue survival of offspring of Plg-RKT−/− females [43]. Moreover, the severity and histological basis of the lactation defect in Plg-RKT−/− mice is much more pronounced than that of plasminogen deficient mice [76, 77]. Phenotypic differences include: 1) All offspring of Plg-RKT−/− females die by postpartum day 2, whereas 60% of offspring of primiparous plasminogen deficient females are alive at postpartum day 2 and the rate of total pup death is gradual, with at least 25% pup survival for 17 days [76]; 2) Lobuloalveolar development takes place normally in plasminogen deficient mice and mammary gland histology is indistinguishable from wild type littermates at postpartum day 2; and 3) milk production in plasminogen deficient mammary glands is normal [76, 77]. Thus, although blockade of ducts with fibrin may play a role, it cannot account for the complete failure of lactation and impaired alveologenesis in Plg-RKT−/− mice. It is also noteworthy that, although uPAR associates with Plg-RKT [34, 37], we are unaware of reports of a lactation defect in uPAR deficient mice.
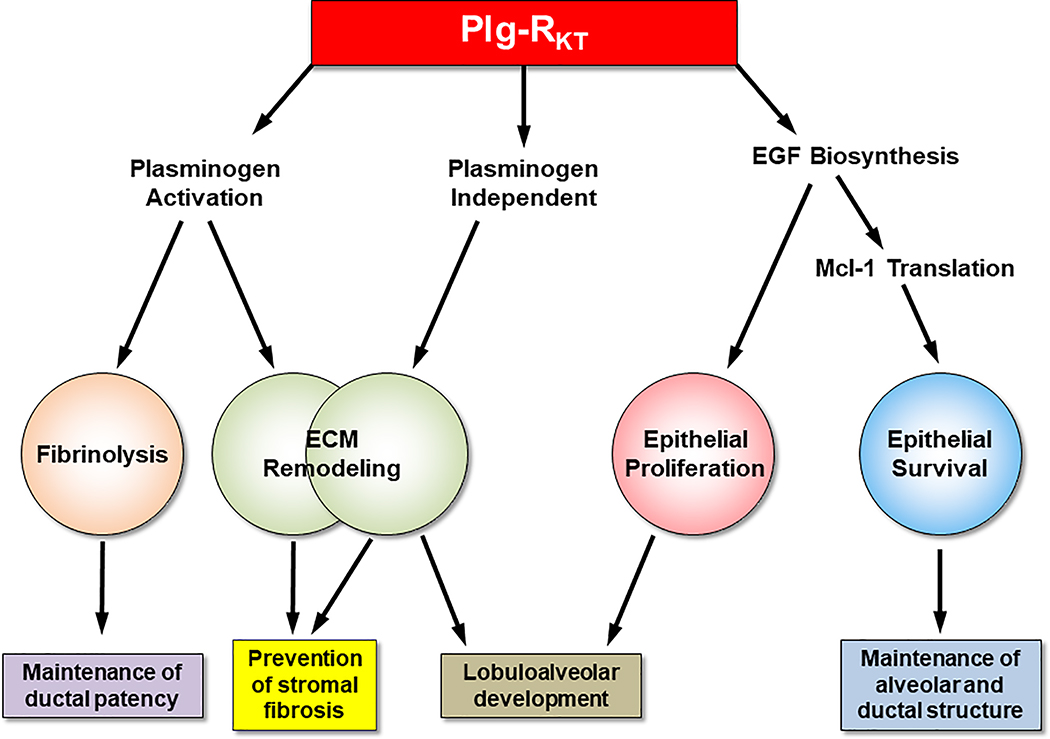
Based on comparison of the lactational phenotype published for plasminogen deficient mice [76, 77] with results with Plg-RKT−/− mice, the influence of Plg-RKT on ECM remodeling can occur by both plasminogen-dependent and plasminogen-independent mechanisms (Fig. 8). By promoting plasminogen activation, Plg-RKT can exert anti-fibrotic effects by regulating remodeling of the stromal fibrin scaffold, activation of MMP’s for collagen remodeling, and proteolysis of the ECM component, entactin/nidogen-1. Plg-RKT may also exert plasminogen-independent anti-fibrotic effects as mammary gland fibrosis is observed at much earlier time points in lactational development of Plg-RKT−/− glands compared with plasminogen deficient glands [76]. Plg-RKT may also regulate plasminogen-independent ECM remodeling for lobuloalveolar development as lobuloalveolar development proceeds normally in plasminogen deficient glands [76, 77]. In addition, Plg-RKT promotes EGF biosynthesis, in a plasminogen-independent fashion, to allow proliferation of epithelial cells to promote lobuloalveolar development. Furthermore, via promoting EGF biosynthesis, Plg-RKT promotes Mcl-1 translation to inhibit apoptosis and maintain alveolar and ductal structure. Finally, Plg-RKT can promote plasminogen activation on the surfaces of luminal epithelial cells for fibrin surveillance within the alveoli and ducts to maintain alveolar patency. Plg-RKT is, thus, a critical protein required for survival of the species, based on the total failure of lactation in Plg-RKT−/−mice. We speculate that this may have major implications for the function of other organs that undergo post-embryonic morphogenesis including kidney [78], prostate [79], lacrimal gland [80], and salivary gland [81] in that Plg-RKT may regulate morphogenesis, particularly epitheliogenesis, in response to pathological challenges.
Figure 8. Working model for regulation of lactogenesis by Plg-RKT.
Plg-RKT regulates key steps essential for nomal lactogenesis. By promoting plasminogen activation, Plg-RKT can exert anti-fibrotic effects by regulating remodeling of the stromal fibrin scaffold, activation of MMP’s for collagen remodeling, and proteolysis of the ECM component, entactin/nidogen-1. Plg-RKT may also exert plasminogen-independent anti-fibrotic effects as mammary gland fibrosis is observed at much earlier time points in lactational development of Plg-RKT−/− glands compared with Plg−/− glands. Plg-RKT may also regulate plasminogen-independent ECM remodeling for lobuloalveolar development as lobuloalveolar development proceeds normally in Plg−/− glands. In addition, Plg-RKT promotes EGF biosynthesis, in a plasminogen-independent fashion, to allow proliferation of epithelial cells to promote lobuloalveolar development. Furthermore, via promoting EGF biosynthesis, Plg-RKT promotes Mcl-1 translation to inhibit apoptosis and maintain alveolar and ductal structure. [Epithelial proliferation and apoptosis are markedly affected by Plg-RKT deletion but are not affected in Plg−/− glands at day 2 of lactation ]. Finally, Plg-RKT can promote plasminogen activation on the surfaces of luminal epithelial cells for fibrin surveillance within the alveoli and ducts to maintain alveolar patency. This diagram has not been published previously.
7. Emerging roles of Plg-RKT
Plg-RKT is likely to play key roles in the many physiological and pathological processes summarized in section 2.2. Here we highlight emerging roles of Plg-RKT for which published information is available.
7.1. Lipoprotein(a) [Lp(a)] catabolism
Lp(a) is an LDL-like particle in which apolipoprotein B-100 is covalently bound to an unique lipoprotein, apolipoprotein(a) [apo(a)]. Lp(a) is both a risk factor for and a factor causing cardiovascular disease and calcific aortic valve stenosis [82]. The sequence of apo(a) is remarkably homologous to plasminogen [83, 84] and Lp(a) competes with plasminogen for the interaction with cell surfaces [85, 86]. Recent in vitro studies in which Plg-RKT was either knocked down or overexpressed in liver cells, have indicated that Plg-RKT is responsible for the majority of Lp(a) uptake in these cells. Interestingly, after Lp(a) is internalized, the apo(a) moiety then trafficks to recycling endosomes with subsequent apo(a) resecretion, while the LDL particle trafficks to lysosomes where it is degraded [87, 88] ( and commentary [89]). Future studies with Plg-RKT−/− mice should aid in elucidating the role of Plg-RKT in Lp(a) metabolism in vivo.
7.2. Neuroendocrine Roles of Plg-RKT
7.2.1. Neurotransmitter release
Catecholaminergic cells, including chromaffin cells of the adrenal medulla and other neurosecretory cells, bind both plasminogen and t-PA, resulting in promotion of plasminogen activation on these cell surfaces [90]. Furthermore, both t-PA and plasminogen are secreted by catecholaminergic cells in response to stimulation with specific secretagogues [91, 92], providing a local neurosecretory environment in which plasmin activity is produced (reviewed in [93]). The locally generated plasmin functions in extracellular processing of secreted hormones [90]. As an example of extracellular plasmic processing, neurotransmitter release from catecholaminergic cells is negatively regulated by cleavage products that are produced by plasmic proteolysis of the major secretory vesicle core protein, Chromogranin A (CgA) [90, 94].
Plg-RKT is prominently expressed in catecholaminergic cells including adrenal medullary chromaffin cells within both murine and human adrenal tissue, as well as in bovine adrenal chromaffin cells, human pheochromocytoma, rat PC12 pheochromocytoma cells and in murine hippocampus [37]. Overexpression of Plg-RKT results in enhanced plasminogen activation on PC12 cells and, importantly, release of the neurotransmitter, norepinephrine is markedly suppressed [37]. Thus, Plg-RKT promotes plasminogen activation to release peptides that feed back to regulate release of catecholamines by these cells and this has major implications for regulation of sympathoadrenal activation and stress responses [93]. Because Plg-RKT is broadly expressed in neuroendocrine and neuronal tissues, these studies additionally suggest a broader paradigm for regulation of local proteolysis and, consequently, neurotransmitter release at many neuroendocrine and neuronal sites.
7.2.2. The inflammatory response in Alzheimer’s Disease
Inflammation plays a key role in Alzheimer’s Disease. The role of microglial activation and macrophage activation in the progression of Alzheimer’s Disease is being increasingly recognized [95]. The neuroimmune response exhibits neuroprotective effects by phagocytosis and clearance of Aβ from the brain. However, activated microglia and macrophages produce neurotoxic cytokines, chemokines, and reactive oxygen species that lead to neuronal death in the CNS [96, 97]. Thus, chronic activation of the neuroimmune system overwhelms its neuroprotective effects by eliciting proinflammatory responses.
Recent studies show that conditional depletion of plasminogen in peripheral blood is highly protective from Aβ deposition and the neuroinflammatory response and is accompanied by decreased microgial/macrophage activation in the 5XFAD murine model of Alzheimer’s Disease [98]. Notably the presence of perivascular macrophages in major vessels of the brain is decreased in plasminogen-depleted Alzheimer’s Disease mice. These studies suggest a previously unappreciated role of circulating plasminogen in Alzheimer’s Disease [98]. Furthermore, conditional depletion of plasminogen reduces migration of perivascular macrophages into the CNS in response to systemic injection of LPS [99]. Blood cells are the source of perivascular macrophages in the CNS [100] and perivascular macrophages show high expression of Plg-RKT [99], suggesting a role for Plg-RKT in plasminogen-dependent macrophage migration in neuroinflammation.
8. Conclusions
This review has focused on recently published studies that have revealed in vivo functions of Plg-RKT. Physiologic and pathophysiologic functions of this plasminogen receptor are based on its ability to bind plasminogen, promote plasminogen activation, localize plasmin on cell surfaces and mediate plasmin-dependent cell signaling. In addition, Plg-RKT may exert plasminogen-independent functions as exemplified by its role in lactational development. Given the predominant role of Plg-RKT in the inflammatory response, future studies of its role in pathophysiologic events involving macrophage and lymphocyte function are warranted. In view of the broad tissue expression of Plg-RKT, its roles in a broad array of pathologies involving other tissues should be revealed in future studies.
Acknowledgments
This work was supported by the National Institutes of Health (grants HL081046 to L.A.M. and HL149511 to R.J.P. and L.A.M.), grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior- Brasil (CAPES)- Finance code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG, Brazil), and by Merit Review Awards #5I01BX002026 and 5I01BX003933 from the U.S. Department of Veterans Affairs (to R.J.P.).
Footnotes
Disclosure of Conflicts of Interest
The authors state that they have no conflict of interest.
References
- 1.Miles LA, Plow EF. Binding and activation of plasminogen on the platelet surface. J Biol Chem. 1985; 260: 4303–11. [PubMed] [Google Scholar]
- 2.Miles LA, Parmer RJ. Plasminogen receptors: the first quarter century. Semin Thromb Hemost. 2013; 39: 329–37. 10.1055/s-0033-1334483 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miles LA, Lighvani S, Baik N, Parmer CM, Khaldoyanidi S, Mueller BM, Parmer RJ. New insights into the role of Plg-RKT in macrophage recruitment. Int Rev Cell Mol Biol. 2014; 309: 259–302. B978–0-12–800255-1.00005–3 [pii]; 10.1016/B978-0-12-800255-1.00005-3 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoylaerts M, Rijken DC, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. J Biol Chem. 1982; 257: 2912–9. [PubMed] [Google Scholar]
- 5.Han J, Baik N, Kim KH, Yang JM, Han GW, Gong Y, Jardi M, Castellino FJ, Felez J, Parmer RJ, Miles LA. Monoclonal antibodies detect receptor-induced binding sites in Glu-plasminogen. Blood. 2011; 118: 1653–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong Y, Kim S-O, Felez J, Grella DK, Castellino FJ, Miles LA. Conversion of glu-plasminogen to lys-plasminogen is necessary for optimal stimulation of plasminogen activation on the endothelial cell surface. J Biol Chem. 2001; 276: 19078–83. [DOI] [PubMed] [Google Scholar]
- 7.Miles LA, Castellino FJ, Gong Y. Critical role for conversion of glu-plasminogen to Lys-plasminogen for optimal stimulation of plasminogen activation on cell surfaces. Trends Cardiovasc Med. 2003; 13: 21–30. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Gong Y, Grella DK, Castellino FJ, Miles LA. Endogenous plasmin converts Glu-plasminogen to Lys-plasminogen on the monocytoid cell surface. J Thromb Haemost. 2003; 1: 1264–70. [DOI] [PubMed] [Google Scholar]
- 9.Félez J, Miles LA, Fábregas P, Jardi M, Plow EF, Lijnen RH. Characterization of cellular binding sites and interactive regions within reactants required for enhancement of plasminogen activation by tPA on the surface of leukocytic cells. Thromb Haemost. 1996; 76: 577–84. [PubMed] [Google Scholar]
- 10.Plow EF, Freaney DE, Plescia J, Miles LA. The plasminogen system and cell surfaces: Evidence for plasminogen and urokinase receptors on the same cell type. J Cell Biol. 1986; 103: 2411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall SW, Humphries JE, Gonias SL. Inhibition of cell surface receptor-bound plasmin by α2- antiplasmin and α2-macroglobulin. J Biol Chem. 1991; 266: 12329–36. [PubMed] [Google Scholar]
- 12.Lu BG, Sofian T, Law RH, Coughlin PB, Horvath AJ. Contribution of conserved lysine residues in the alpha2-antiplasmin C terminus to plasmin binding and inhibition. J Biol Chem. 2011; 286: 24544–52. 10.1074/jbc.M111.229013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Syrovets T, Simmet T. The Serine Protease Plasmin Triggers Expression of the CC-Chemokine Ligand 20 in Dendritic Cells via Akt/NF-kappaB-Dependent Pathways. J Biomed Biotechnol. 2012; 2012: 186710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Syrovets T, Lunov O, Simmet T. Plasmin as a proinflammatory cell activator. J Leukoc Biol. 2012; 92: 509–19. [DOI] [PubMed] [Google Scholar]
- 15.Miles LA, Levin EG, Plescia J, Collen D, Plow EF. Plasminogen receptors, urokinase receptors and their modulation on human endothelial cells. Blood. 1988; 72: 628–35. [PubMed] [Google Scholar]
- 16.Miles LA, Dahlberg CM, Plescia J, Felez J, Kato K, Plow EF. Role of cell-surface lysines in plasminogen binding to cells: Identification of alpha-Enolase as a candidate plasminogen receptor. Biochemistry. 1991; 30: 1682–91. [DOI] [PubMed] [Google Scholar]
- 17.Redlitz A, Fowler BJ, Plow EF, Miles LA. The role of an enolase-related molecule in plasminogen binding to cells. Eur J Biochem. 1995; 227: 407–15. [DOI] [PubMed] [Google Scholar]
- 18.Hembrough TA, Vasudevan J, Allietta MM, Glass WF, Gonias SL. A cytokeratin 8-like protein with plasminogen-binding activity is present on the external surfaces of hepatocytes, HepG2 cells and breast carcinoma cell lines. J Cell Sci. 1995; 108 ( Pt 3): 1071–82. [DOI] [PubMed] [Google Scholar]
- 19.Hembrough TA, Kralovich KR, Li L, Gonias SL. Cytokeratin 8 released by breast carcinoma cells in vitro binds plasminogen and tissue-type plasminogen activator and promotes plasminogen activation. Biochem J. 1996; 317: 763–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kassam G, Le BH, Choi KS, Kang HM, Fitzpatrick SL, Louie P, Waisman DM. The p11 subunit of the annexin II tetramer plays a key role in the stimulation of t-PA-dependent plasminogen activation. Biochemistry. 1998; 37: 16958–66. [DOI] [PubMed] [Google Scholar]
- 21.Hawley SB, Tamura T, Miles LA. Purification, cloning, and characterization of a profibrinolytic plasminogen-binding protein, TIP49a. J Biol Chem. 2001; 276: 179–86. [DOI] [PubMed] [Google Scholar]
- 22.Herren T, Burke TA, Das R, Plow EF. Identification of histone H2B as a regulated plasminogen receptor. Biochemistry. 2006; 45: 9463–74. [DOI] [PubMed] [Google Scholar]
- 23.Dudani AK, Ganz PR. Endothelial cell surface actin serves as a binding site for plasminogen, tissue plasminogen activator and lipoprotein(a). Br J Haematol. 1996; 95: 168–78. [DOI] [PubMed] [Google Scholar]
- 24.Miles LA, Andronicos NM, Baik N, Parmer RJ. Cell-surface actin binds plasminogen and modulates neurotransmitter release from catecholaminergic cells. J Neurosci. 2006; 26: 13017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briens A, Bardou I, Miles LA, Parmer RJ, Vivien D, Docagne F. Astrocytes regulate the balance between plasminogen acivation and plasmin clearance via cell-surface actin. Submitted to Cell Research. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan Z, Larson PJ, Bognacki J, Raghunath PN, Tomaszewski JE, Kuo A, Canziani G, Chaiken I, Cines DB, Higazi AA. Tissue factor regulates plasminogen binding and activation. Blood. 1998; 91: 1987–98. [PubMed] [Google Scholar]
- 27.Miles LA, Dahlberg CM, Levin EG, Plow EF. Gangliosides interact directly with plasminogen and urokinase and may mediate binding of these components to cells. Biochemistry. 1989; 280: 9337–43. [DOI] [PubMed] [Google Scholar]
- 28.Miles LA, Ginsberg MH, White JG, Plow EF. Plasminogen interacts with human platelets through two distinct mechanisms. J Clin Invest. 1986; 77: 2001–9. 10.1172/JCI112529 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pluskota E, Soloviev DA, Bdeir K, Cines DB, Plow EF. Integrin alphaMbeta2 orchestrates and accelerates plasminogen activation and fibrinolysis by neutrophils. J Biol Chem. 2004; 279: 18063–72. [DOI] [PubMed] [Google Scholar]
- 30.Lishko VK, Novokhatny VV, Yakubenko VP, Skomorovska-Prokvolit HV, Ugarova TP. Characterization of plasminogen as an adhesive ligand for integrins {alpha}M{beta}2 (Mac-1) and {alpha}5{beta}1(VLA-5). Blood. 2004. [DOI] [PubMed] [Google Scholar]
- 31.Parkkinen J, Raulo E, merenmies J, Nolo R, Kajander EO, Baumann M, Rauvala H. Amphoterin, the 30-kDa protein in a family of HMG1-type polypeptides. Enhanced expression in transformed cells, leading edge localization, and interactions with plasminogen activation. J Biol Chem. 1993; 268: 19726–38. [PubMed] [Google Scholar]
- 32.Kanalas JJ, Makker SP. Identification of the rat Heymann nephritis autoantigen (GP330) as a receptor site for plasminogen. J Biol Chem. 1991; 266: 10825–9. [PubMed] [Google Scholar]
- 33.Miles LA, Plow EF, Waisman DM, Parmer RJ. Plasminogen receptors. J Biomed Biotechnol. 2012; 2012: 130735 10.1155/2012/130735 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andronicos NM, Chen EI, Baik N, Bai H, Parmer CM, Kiosses WB, Kamps MP, Yates JR III, Parmer RJ, Miles LA. Proteomics-based discovery of a novel, structurally unique, and developmentally regulated plasminogen receptor, Plg-RKT, a major regulator of cell surface plasminogen activation. Blood. 2010; 115: 1319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miles LA, Andronicos NM, Chen EI, Baik N, Bai H, Parmer CM, Lighvani S, Nangia S, Kiosses WB, Kamps MP, Yates JR III, Parmer RJ. Identification of the novel plasminogen receptor, Plg-RKT. In: Man TK, Flores RJ, eds. Proteomics/Book 1: Human Diseases and Protein Functions, ISBN 978–953-307–832-8 edn: Intech, 2012, 219–38. [Google Scholar]
- 36.Liu M, Zhang S. A kringle-containing protease with plasminogen-like activity in the basal chordate Branchiostoma belcheri. Biosci Rep. 2009; 29: 385–95. [DOI] [PubMed] [Google Scholar]
- 37.Bai H, Baik N, Kiosses WB, Krajewski S, Miles LA, Parmer RJ. The novel plasminogen receptor, plasminogen receptor(KT) (Plg-R(KT)), regulates catecholamine release. J Biol Chem. 2011; 286: 33125–33. M111.218693 [pii]; 10.1074/jbc.M111.218693 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miles LA, Lighvani S, Baik N, Andronicos NM, Chen EI, Parmer CM, Khaldoyanidi S, Diggs JE, Kiosses WB, Kamps MP, Yates JR III, Parmer RJ. The plasminogen receptor, Plg-R(KT), and macrophage function. J Biomed Biotechnol. 2012; 2012: 250464 10.1155/2012/250464 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thaler B, Baik N, Hohensinner PJ, Baumgartner J, Panzenbock A, Stojkovic S, Demyanets S, Huk I, Rega-Kaun G, Kaun C, Prager M, Fischer MB, Huber K, Speidl WS, Parmer RJ, Miles LA, Wojta J. Differential expression of Plg-RKT and its effects on migration of proinflammatory monocyte and macrophage subsets. Blood. 2019; 134: 561–7. 10.1182/blood.2018850420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lighvani S, Baik N, Diggs JE, Khaldoyanidi S, Parmer RJ, Miles LA. Regulation of macrophage migration by a novel plasminogen receptor Plg-RKT. Blood. 2011; 118: 5622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Felez J, Chanquia CJ, Fabregas P, Plow EF, Miles LA. Competition between plasminogen and tissue plasminogen activator for cellular binding sites. Blood. 1993; 82: 2433–41. [PubMed] [Google Scholar]
- 42.Miles LA, Krajewski S, Baik N, Booth NA, Mueller BM, Kiosses WB, Bai H, Nangia S, Parmer RJ. Plg-PKT Protein is Broadly Distributed in Human and Murine Tissues. Presented at the 22nd International Congress on Fibronolysis and Proteolysis, Marseille, France 2014: (CP39). [Google Scholar]
- 43.Miles LA, Baik N, Bai H, Makarenkova HP, Kiosses WB, Krajewski S, Castellino FJ, Valenzuela A, Varki NM, Mueller BM, Parmer RJ. The Plasminogen Receptor, Plg-RKT, is Essential for Mammary Lobuloalveolar Development and Lactation. J Thromb Haemost. 2018; 16: 919–32. 10.1111/jth.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sousa LP, Alessandri AL, Pinho V, Teixeira MM. Pharmacological strategies to resolve acute inflammation. Curr Opin Pharmacol. 2013; 13: 625–31. 10.1016/j.coph.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LA, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007; 21: 325–32. 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ploplis VA, French EL, Carmeliet P, Collen D, Plow EF. Plasminogen deficiency differentially affects recruitment of inflammatory cell populations in mice. Blood. 1998; 91: 2005–9. [PubMed] [Google Scholar]
- 47.Plow EF, Ploplis VA, Busuttil S, Carmeliet P, Collen D. A role of plasminogen in atherosclerosis and restenosis models in mice. Thromb Haemost. 1999; 82 Suppl 1: 4–7. [PubMed] [Google Scholar]
- 48.Busuttil SJ, Ploplis VA, Castellino FJ, Tang L, Eaton JW, Plow EF. A central role for plasminogen in the inflammatory response to biomaterials. J Thromb Haemost. 2004; 2: 1798–805. [DOI] [PubMed] [Google Scholar]
- 49.Gong Y, Hart E, Shchurin A, Hoover-Plow J. Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J Clin Invest. 2008; 118: 3012–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swaisgood CM, Schmitt D, Eaton D, Plow EF. In vivo regulation of plasminogen function by plasma carboxypeptidase B. J Clin Invest. 2002; 110: 1275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ploplis V, French E, Carmeliet P, Collen D. The plasminogen system and cell migration during an inflammatory response. Fibrinolysis. 1996; in press. [Google Scholar]
- 52.Carmo AA, Costa BR, Vago JP, de Oliveira LC, Tavares LP, Nogueira CR, Ribeiro AL, Garcia CC, Barbosa AS, Brasil BS, Dusse LM, Barcelos LS, Bonjardim CA, Teixeira MM, Sousa LP. Plasmin induces in vivo monocyte recruitment through protease-activated receptor-1-, MEK/ERK-, and CCR2-mediated signaling. J Immunol. 2014; 193: 3654–63. 10.4049/jimmunol.1400334. [DOI] [PubMed] [Google Scholar]
- 53.Sugimoto MA, Ribeiro AL, Costa BR, Vago JP, Lima KM, Carneiro FS, Ortiz MM, Lima GL, Carmo AA, Rocha RM, Perez DA, Reis AC, Pinho V, Miles LA, Garcia CC, Teixeira MM, Sousa LP. Plasmin and Plasminogen induce macrophage reprogramming and regulate key steps of inflammation resolution via Annexin A1. Blood. 2017. 10.1182/blood-2016-09-742825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vago JP, Sugimoto MA, Lima KM, Negreiros-Lima GL, Baik N, Teixeira MM, Perretti M, Parmer RJ, Miles LA, Sousa LP. Plasminogen and the Plasminogen Receptor, Plg-RKT, Regulate Macrophage Phenotypic, and Functional Changes. Front Immunol. 2019; 10: 1458 10.3389/fimmu.2019.01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herren T, Burke TA, Jardi M, Felez J, Plow EF. Regulation of plasminogen binding to neutrophils. Blood. 2001; 97: 1070–8. [DOI] [PubMed] [Google Scholar]
- 56.Miles LA, Baik N, Lighvani S, Khaldoyanidi S, Varki NM, Bai H, Mueller BM, Parmer RJ. Deficiency of plasminogen receptor, Plg-RKT, causes defects in plasminogen binding and inflammatory macrophage recruitment in vivo. J Thromb Haemost. 2017; 15: 155–62. 10.1111/jth.13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sousa LP, Lopes F, Silva DM, Tavares LP, Vieira AT, Rezende BM, Carmo AF, Russo RC, Garcia CC, Bonjardim CA, Alessandri AL, Rossi AG, Pinho V, Teixeira MM. PDE4 inhibition drives resolution of neutrophilic inflammation by inducing apoptosis in a PKA-PI3K/Akt-dependent and NF-kappaB-independent manner. J Leukoc Biol. 2010; 87: 895–904. 10.1189/jlb.0809540. [DOI] [PubMed] [Google Scholar]
- 58.Vago JP, Nogueira CR, Tavares LP, Soriani FM, Lopes F, Russo RC, Pinho V, Teixeira MM, Sousa LP. Annexin A1 modulates natural and glucocorticoid-induced resolution of inflammation by enhancing neutrophil apoptosis. J Leukoc Biol. 2012; 92: 249–58. 10.1189/jlb.0112008. [DOI] [PubMed] [Google Scholar]
- 59.Syrovets T, Jendrach M, Rohwedder A, Schule A, Simmet T. Plasmin-induced expression of cytokines and tissue factor in human monocytes involves AP-1 and IKKbeta-mediated NF-kappaB activation. Blood. 2001; 97: 3941–50. [DOI] [PubMed] [Google Scholar]
- 60.Li Q, Laumonnier Y, Syrovets T, Simmet T. Plasmin triggers cytokine induction in human monocyte-derived macrophages. Arterioscler Thromb Vasc Biol. 2007; 27: 1383–9. [DOI] [PubMed] [Google Scholar]
- 61.Zalfa C, Azmoon P, Mantuano E, Gonias SL. Tissue-type plasminogen activator neutralizes LPS but not protease-activated receptor-mediated inflammatory responses to plasmin. J Leukoc Biol. 2019; 105: 729–40. 10.1002/jlb.3a0818-329rrr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, Crameri R, Duan S, Eiwegger T, Eljaszewicz A, Ferstl R, Frei R, Garbani M, Globinska A, Hess L, Huitema C, Kubo T, Komlosi Z, Konieczna P, Kovacs N, Kucuksezer UC, Meyer N, Morita H, Olzhausen J, O’Mahony L, Pezer M, Prati M, Rebane A, Rhyner C, Rinaldi A, Sokolowska M, Stanic B, Sugita K, Treis A, van de Veen W, Wanke K, Wawrzyniak M, Wawrzyniak P, Wirz OF, Zakzuk JS, Akdis CA. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: Receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016; 138: 984–1010. 10.1016/j.jaci.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 63.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010; 10: 170–81. 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 64.Vago JP, Tavares LP, Garcia CC, Lima KM, Perucci LO, Vieira EL, Nogueira CR, Soriani FM, Martins JO, Silva PM, Gomes KB, Pinho V, Bruscoli S, Riccardi C, Beaulieu E, Morand EF, Teixeira MM, Sousa LP. The role and effects of glucocorticoid-induced leucine zipper in the context of inflammation resolution. J Immunol. 2015; 194: 4940–50. 10.4049/jimmunol.1401722. [DOI] [PubMed] [Google Scholar]
- 65.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014; 41: 14–20. S1074–7613(14)00228–3 [pii]; 10.1016/j.immuni.2014.06.008 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014; 14: 166–80. 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosenwald M, Koppe U, Keppeler H, Sauer G, Hennel R, Ernst A, Blume KE, Peter C, Herrmann M, Belka C, Schulze-Osthoff K, Wesselborg S, Lauber K. Serum-derived plasminogen is activated by apoptotic cells and promotes their phagocytic clearance. J Immunol. 2012; 189: 5722–8. 10.4049/jimmunol.1200922. [DOI] [PubMed] [Google Scholar]
- 68.Das R, Ganapathy S, Settle M, Plow EF. Plasminogen promotes macrophage phagocytosis in mice. Blood. 2014; 124: 679–88. blood-2014–01-549659 [pii]; 10.1182/blood-2014-01-549659 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miles LA, Parmer RJ. Setting the table for macrophages. Blood. 2014; 124: 665–6. 124/5/665 [pii]; 10.1182/blood-2014-06-579631 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Borg RJ, Samson AL, Au AE, Scholzen A, Fuchsberger M, Kong YY, Freeman R, Mifsud NA, Plebanski M, Medcalf RL. Dendritic Cell-Mediated Phagocytosis but Not Immune Activation Is Enhanced by Plasmin. PLoS One. 2015; 10: e0131216 10.1371/journal.pone.0131216 [doi];PONE-D-15–05967 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma Q, Ozel AB, Ramdas S, McGee B, Khoriaty R, Siemieniak D, Li HD, Guan Y, Brody LC, Mills JL, Molloy AM, Ginsburg D, Li JZ, Desch KC. Genetic variants in PLG, LPA, and SIGLEC 14 as well as smoking contribute to plasma plasminogen levels. Blood. 2014; 124: 3155–64. blood-2014–03-560086 [pii]; 10.1182/blood-2014-03-560086 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991; 115: 1383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995; 267: 891–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pullan S, Wilson J, Metcalfe A, Edwards GM, Goberdhan N, Tilly J, Hickman JA, Dive C, Streuli CH. Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium. J Cell Sci. 1996; 109 ( Pt 3): 631–42. [DOI] [PubMed] [Google Scholar]
- 75.Fu NY, Rios AC, Pal B, Soetanto R, Lun AT, Liu K, Beck T, Best SA, Vaillant F, Bouillet P, Strasser A, Preiss T, Smyth GK, Lindeman GJ, Visvader JE. EGF-mediated induction of Mcl-1 at the switch to lactation is essential for alveolar cell survival. Nat Cell Biol. 2015; 17: 365–75. 10.1038/ncb3117. [DOI] [PubMed] [Google Scholar]
- 76.Green KA, Nielsen BS, Castellino FJ, Romer J, Lund LR. Lack of plasminogen leads to milk stasis and premature mammary gland involution during lactation. Dev Biol. 2006; 299: 164–75. [DOI] [PubMed] [Google Scholar]
- 77.Lund LR, Bjorn SF, Sternlicht MD, Nielsen BS, Solberg H, Usher PA, Osterby R, Christensen IJ, Stephens RW, Bugge TH, Dano K, Werb Z. Lactational competence and involution of the mouse mammary gland require plasminogen. Development. 2000; 127: 4481–92. [DOI] [PubMed] [Google Scholar]
- 78.Shah MM, Sampogna RV, Sakurai H, Bush KT, Nigam SK. Branching morphogenesis and kidney disease. Development. 2004; 131: 1449–62. 10.1242/dev.01089. [DOI] [PubMed] [Google Scholar]
- 79.Thomson AA, Marker PC. Branching morphogenesis in the prostate gland and seminal vesicles. Differentiation. 2006; 74: 382–92. 10.1111/j.1432-0436.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- 80.Gromova A, Voronov DA, Yoshida M, Thotakura S, Meech R, Dartt DA, Makarenkova HP. Lacrimal Gland Repair Using Progenitor Cells. Stem cells translational medicine. 2017; 6: 88–98. 10.5966/sctm.2016-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patel VN, Rebustini IT, Hoffman MP. Salivary gland branching morphogenesis. Differentiation. 2006; 74: 349–64. 10.1111/j.1432-0436.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- 82.Tsimikas S A Test in Context: Lipoprotein(a): Diagnosis, Prognosis, Controversies, and Emerging Therapies. J Am Coll Cardiol. 2017; 69: 692–711. 10.1016/j.jacc.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 83.Eaton DL, Fless GM, Kohr WJ, McLean JW, Xu QT, Miller CG, Lawn RM, Scanu AM. Partial amino acid sequence of apolipoprotein(a) shows that it is homologous to plasminogen. Proc Natl Acad Sci U S A. 1987; 84: 3224–8. 10.1073/pnas.84.10.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McLean JW, Tomlinson JE, Kuang WJ, Eaton DL, Chen EY, Fless GM, Scanu AM, Lawn RM. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 1987; 330: 132–7. 10.1038/330132a0. [DOI] [PubMed] [Google Scholar]
- 85.Miles LA, Fless GM, Levin EG, Scanu AM, Plow EF. A potential basis for the thrombotic risks associated with lipoprotein(a). Nature. 1989; 339: 301–3. [DOI] [PubMed] [Google Scholar]
- 86.Hajjar KA, Gavish D, Breslow JL, Nachman RL. Lipoprotein(a) modulation of endothelial cell surface fibrinolysis and its potential role in atherosclerosis. Nature. 1989; 339: 303–5. [DOI] [PubMed] [Google Scholar]
- 87.Sharma M, Redpath GM, Williams MJ, McCormick SP. Recycling of Apolipoprotein(a) After PlgRKT-Mediated Endocytosis of Lipoprotein(a). Circ Res. 2017; 120: 1091–102. 10.1161/circresaha.116.310272. [DOI] [PubMed] [Google Scholar]
- 88.McCormick SPA, Schneider WJ. Lipoprotein(a) catabolism: a case of multiple receptors. Pathology. 2019; 51: 155–64. 10.1016/j.pathol.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 89.Yeang C, Gordts PL, Tsimikas S. Novel Lipoprotein(a) Catabolism Pathway via Apolipoprotein(a) Recycling: Adding the Plasminogen Receptor PlgRKT to the List. Circ Res. 2017; 120: 1050–2. 10.1161/circresaha.117.310700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Parmer RJ, Mahata M, Gong Y, Mahata S, Jiang Q, O’Connor DT, Xi X-P, Miles LA. Processing of chromogranin A by plasmin provides a novel mechanism for regulating catecholamine secretion. J Clin Invest. 2000; 106: 907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parmer RJ, Mahata M, Mahata S, Sebald MT, O’Connor DT, Miles LA. Tissue plasminogen activator (t-PA) is targeted to the regulated secretory pathway: Catecholamine storage vesicles as a reservoir for the rapid release of t-PA. J Biol Chem. 1997; 272: 1976–82. [DOI] [PubMed] [Google Scholar]
- 92.Gualandris A, Jones TE, Strickland S, Tsirka SE. Membrane depolarization induces calcium-dependent secretion of tissue plasminogen activator. J Neurol Sci. 1996; 16: 2220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bai H, Nangia S, Parmer RJ. The plasminogen activation system and the regulation of catecholaminergic function. J Biomed Biotechnol. 2012; 2012: 721657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiang Q, Taupenot L, Mahata SK, Mahata M, O’Connor DT, Miles LA, Parmer RJ. Proteolytic cleavage of chromogranin A (CgA) by plasmin. Selective liberation of a specific bioactive CgA fragment that regulates catecholamine release. J Biol Chem. 2001; 276: 25022–9. 10.1074/jbc.M101545200 [doi];M101545200 [pii]. [DOI] [PubMed] [Google Scholar]
- 95.Heneka MT, Golenbock DT, Latz E. Innate immunity in Alzheimer’s disease. Nat Immunol. 2015; 16: 229–36. 10.1038/ni.3102. [DOI] [PubMed] [Google Scholar]
- 96.Ramesh G, MacLean AG, Philipp MT. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm. 2013; 2013: 480739 10.1155/2013/480739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hansen DV, Hanson JE, Sheng M. Microglia in Alzheimer’s disease. J Cell Biol. 2018; 217: 459–72. 10.1083/jcb.201709069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baker SK, Chen ZL, Norris EH, Revenko AS, MacLeod AR, Strickland S. Blood-derived plasminogen drives brain inflammation and plaque deposition in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2018; 115: E9687–e96. 10.1073/pnas.1811172115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baker SK, Chen ZL, Norris EH, Strickland S. Plasminogen mediates communication between the peripheral and central immune systems during systemic immune challenge with lipopolysaccharide. J Neuroinflammation. 2019; 16: 172 10.1186/s12974-019-1560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Soulas C, Donahue RE, Dunbar CE, Persons DA, Alvarez X, Williams KC. Genetically modified CD34+ hematopoietic stem cells contribute to turnover of brain perivascular macrophages in long-term repopulated primates. Am J Pathol. 2009; 174: 1808–17. 10.2353/ajpath.2009.081010. [DOI] [PMC free article] [PubMed] [Google Scholar]