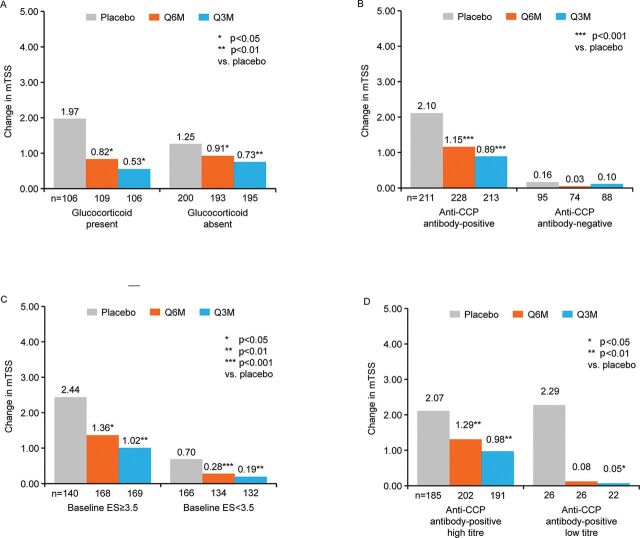
Figure 2.
Change in mTSS by glucocorticoid status (A), anti-CCP antibody status (B), baseline ES (C) and anti-CCP antibody-positive titres (high/low) (D) of the total group by pooled analysis for up to 12 months. CCP, cyclic citrullinated peptide; ES, bone erosion score; mTSS, modified total Sharp score; Q3M, denosumab 60 mg every 3 months; Q6M, denosumab 60 mg every 6 months. Data are means with 95% CIs. *p<0.05; **p<0.01; ***p<0.001 vs placebo.