Abstract
Objective
To appraise studies reporting on clinical effectiveness and safety of surgical meshes used to augment rotator cuff repairs (RCRs).
Design
Systematic review and meta-analysis.
Data sources
MEDLINE, Embase and Cochrane databases were searched between April 2006 and April 2020.
Eligibility criteria
All studies evaluating adults (≥18 years) undergoing RCR were considered. There were no language restrictions.
Data extraction and synthesis
Screening, data extraction and quality appraisal were conducted by two independent reviewers. Meta-analysis was conducted using a random-effects models if ≥2 comparative studies reported the same outcome measure. Risk of bias assessment was undertaken for randomised (RoB2, Cochrane) and comparative studies (ROBINS-I, Cochrane).
Results
We included 60 studies, consisting of 7 randomised controlled trials, 13 observational comparative studies and 40 observational case series. All comparative studies reported on shoulder-specific functional outcome scores, 18 on the radiographic occurrence of re-tear and 14 on pain score metrics. All studies contained some risk of bias.
Compared with non-augmented repair, a small improvement in shoulder-specific function or pain scores was observed for synthetic patches with a mean improvement of 6.7 points on the University of California Los Angles (UCLA) shoulder score (95% CI 0.1 to 13.4) and 0.46 point reduction on the Visual Analogue Scale (95% CI −0.74 to −0.17), respectively. A reduced likelihood of radiologically observed re-tear was observed for synthetic (risk ratio (RR) 0.41, 95% CI 0.27 to 0.61) and allograft (RR 0.34, 95% CI 0.18 to 0.65) patches. A total of 49 studies reported on the occurrence of complications. Slightly higher crude complication rates were observed following patch-augmented repair (2.1%) than standard repair (1.6%).
Conclusions
While several studies suggest a decreased failure rate and small improvements in shoulder function and pain following augmented RCR, a paucity of rigorous clinical evaluation, for both effectiveness and safety, prevents firm recommendations.
Prospero registration number
CRD42017057908.
Keywords: orthopaedic & trauma surgery, adverse events, biotechnology & bioinformatics
Strengths and limitations of this study.
The largest systematic appraisal of the clinical effectiveness and safety of implantable meshes for augmented rotator cuff repair.
Thorough searching of three major electronic databases and reporting as per Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. The study protocol was published a priori, with inclusion of all non-English language articles.
Study bias and substantial heterogeneity between studies means that our meta-analysis results must be interpreted very cautiously, seriously limiting our ability to draw firm recommendations.
The observed differences in outcomes between patch types could reflect, to some degree, chance findings given the limited numbers of studies and small typical study size. Confirmation of findings by further trials is warranted.
Introduction
Shoulder pain is the third most prevalent musculoskeletal disorder and is responsible for prolonged periods of disability, absence from work and a significant health-economic burden.1–3 Rotator cuff problems account for a large proportion of shoulder pain and results in pain, weakness, reduced shoulder mobility and sleep disturbance.4 It is estimated that the overall prevalence of full-thickness tears is between 15% and 20% with the rate set to increase as populations age.5 6 While some are asymptomatic, many symptomatic full-thickness tears will often require surgical repair, with successful repair correlating with symptom resolution.7 Indeed, 9000 rotator cuff repairs (RCRs) are performed each year in the NHS in England alone, at a cost of £6500 per operation.8 Unfortunately, randomised controlled trials (RCTs) have demonstrated a failure rate of up to 40%, with increasing patient age and tear size, both predictive of failure.7 9 While various surgical techniques have attempted to improve the outcome of RCR, there remains a real need to improve healing rates.
One approach is the use of an implant called a surgical mesh, or patch, to augment the repair. A patch made from Teflon was first described over 30 years ago,10 but recently, the number and types of patch specifically for rotator cuff surgery have increased significantly. These can broadly be divided into categories based on the materials used; xenograft or allograft are decellularised extracellular matrix derived from animal or human cadaveric tissues, respectively; synthetic grafts are materials derived from a variety of bio-inert polymers; and autografts are patient’s own whole tendon harvested from various anatomical sites. Patch augmentation can also be classified based on the method of application into ‘on-lay’ or ‘bridging’. The former refers to the application of a synthetic or biological patch over the top of a standard repair to provide mechanical stability and biological stimulation, reducing the likelihood of failure and improving patient outcomes.11 12 In contrast, ‘bridging’ refers to the use of a patch as an interposition graft to fill any residual defect of an otherwise irreparable tear, providing a scaffold for the regeneration of tendon and/or scar tissue formation.13
Recent and important debate surrounding medical device regulation has emphasised the need for robust evaluations of safety, efficacy and survellience.14 Unfortunately, in the context of patch augmentation, the growing number of available patches, mixed results and recent concerns over safety, including adverse immunological responses,15 have generated a clouded and uncertain landscape.
The aim of this systematic review is to identify and critically appraise those studies reporting on the clinical effectiveness and safety of patch-augmented surgical repair in adults with rotator cuff tears.
Methods
Protocol and registration
The review protocol and search strategy has been previously been registered (PROSPERO Registration: CRD42017057908) and published in full.16
Eligibility criteria
Population
The review incorporated studies of adult (≥18 years) patients who required surgical repair of a rotator cuff tear. No restrictions were applied to tear type (partial or full thickness), size (small through to massive), tendon involvement (supraspinatus, infraspinatus, teres minor or subscapularis), primary or recurrent tears, or the presence of medical comorbidities. For the purpose of this review small (<1 cm), medium (1–3 cm) and large (3–5 cm) tears were classified according to the DeOrio and Cofield classification.17 Due to the large number of classification systems available, tears were considered massive if they met one of following criteria: (1) Measured >5 cm in the anterior-posterior dimension,17 (2) Involved ≥2 tendons,18 or were (3) Described as being massive by the study authors.
Interventions
All studies where at least one treatment arm included the use of patches to augment rotator cuff surgery were included. A patch was defined as an implantable human, synthetic or animal material which is used with the aim of improving tissue healing and/or patient outcome via some form of mechanical support. Patches were grouped into xenograft, allograft, autograft or synthetic. There was no restriction placed on the type of surgery received or the experience of the surgeon. The type of patch surgery was classified as either (1) ‘on-lay’ or (2) ‘bridging’ in accordance with previously reported definitions.19 We excluded studies that investigated the use of sutures or anchors in isolation, or studies investigating drug therapy or physiotherapy, except when used as a comparator group or in addition to patch augmentation.
Comparators
No restriction was placed on the type or number of control groups.
Outcomes
The primary outcomes of interest in this review were: (1) Shoulder-specific function and pain scores—measured using a previously validated scale. (2) Patch-related adverse events (complications). (3) Shoulder pain outcomes—measured using validated tools such as the Visual Analogue Scale (VAS) or other scales. (4) Health-related quality life—measured using tools such as Short Form-36 (SF-36), EuroQol 5-dimension (EQ-5D) Questionnaires or other assessment measures. The main secondary outcome was the radiological assessment of postoperative rotator cuff integrity (re-tear).
Study types
We considered all relevant RCTs and observational studies (comparative and single group) involving at least five patients. No language restrictions were applied. In vitro studies, animal studies, review articles, editorials and studies involving up to five patients were all excluded.
Search strategy
A previous Cochrane review had carried out a comprehensive search prior to April 2006.20 We searched the following databases between the dates of April 2006 and February 2017 (and updated our search in April 2020): (1) MEDLINE, (2) Embase, (3) The Cochrane Library. In addition, the reference list of all identified articles and reviews identified were checked for relevant articles.19 21–24
Study selection
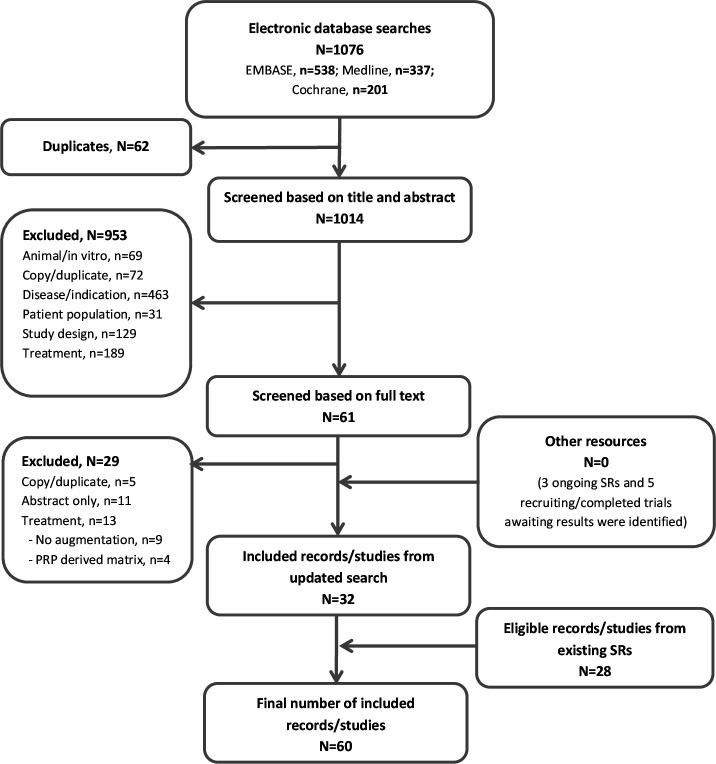
Two authors (MB and NSN) independently screened all titles and abstracts identified from the search strategy. Full reports for all relevant studies identified were then reviewed and assessed against the eligibility criteria. A third independent reviewer (GG) was available to resolve any disagreements regarding study inclusion. Reasons for exclusion are detailed in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram (figure 1).
Figure 1.
PRISMA flow chart of study selection. N, number; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SR, systematic review.
Data extraction
Two authors (MB and NSN) extracted the following data from all eligible studies: general study information (authors, publication year, study location), study population (sample size, age, gender, tear size), study characteristics (study design, inclusion/exclusion criteria, duration of clinical and radiological follow-up, surgical technique, patch characteristics), all primary and secondary outcomes for each study, and adverse events or complications. Each reviewer independently checked the results of the data extraction process.
Risk of bias assessment
The risk of bias was independently assessed by two authors (MB and NSN) and discrepancies discussed with a third reviewer (GG) allowing resolution based on unanimous decision. RCTs were assessed using the isk-of-bias tool for randomized trials (RoB2) provided by the Cochrane Collaboration.25 Observational comparative studies were assessed using the ROBINS-I tool (Risk Of Bias In Non-randomised Studies - of Interventions).26
Data analysis
Identified studies were stratified (RCTs, observational comparative studies, single-arm studies) and a narrative summary of results from RCTs and observational comparative studies reported in accordance with the standards set out in the PRISMA-P checklist.27 Data from single-arm studies were only used in the quantification of complications. All studies that compared the outcomes of RCR with graft augmentation versus standard RCR were considered for meta-analysis. A meta-analysis was conducted only for outcomes consistently reported across studies and forest plots constructed using the R (V.3.2.4) packages ‘meta’ and ‘metafor’. Regardless of the observed statistical heterogeneity, we conducted an analysis for each patch type (xenograft, allograft, autograft or synthetic) when each type was represented by at least two studies. Given the known controversy surrounding xenograft isolated from small intestinal submucosa (SIS), the analysis for xenografts was further divided into SIS-derived and non-SIS. There were insufficient study numbers to permit subgrouping based on graft configuration (on-lay or bridging).
Statistical analysis
For dichotomous parameters included in the meta-analysis the risk ratio (RR) with 95% CI was calculated for each graft type. For continuous variables, such as shoulder-specific functional outcome scores, the effect was reported as the mean difference with 95% CI. Due to significant heterogeneity in the specific functional shoulder scores used between studies, a meta-analysis was conducted using the most frequently used score across all studies at final follow-up. Within each patch type, if no single functional outcome score was consistently used, we combined scores and the standardised mean difference was meta-analysed (95% CI). Studies in which no SD was calculable, or where only subcomponents of functional outcome scores were reported, were excluded. Heterogeneity was characterised by use of the I2 statistic and a random-effect analysis was used to allow for heterogeneity among studies. Meta-analyses were conducted on available data across all comparative studies; sensitivity meta-analyses were then performed to assess the impact of restricting to only one study design where two or more studies of the same design were available.
Patient involvement
Patient representatives were full members of the PARCS Study28 steering committee and provided critical feedback on the systematic review protocol.
Results
Study selection
The search strategy identified 1076 articles, of which 62 were duplicates (figure 1). A total of 1014 abstracts were reviewed in detail with 61 appearing to meet inclusion criteria. After full-text review 29 articles were excluded (details in figure 1). A further 28 articles were identified from existing systematic reviews, generating a total of 60 studies for inclusion, including 2 non-English language articles. The summaries below focus predominantly on the comparative (RCTs and observational) studies.
Study characteristics
Seven RCTs and 13 observational comparative studies analysing 1128 patient events were identified. Most comparative studies assessed a single patch against standard repair, with some studies having up to three treatment arms.29–31 A single study compared autograft patch augmentation to conservative therapy,32 while a further study assessed the effect of xenograft patch with or without mesenchymal stem cell augmentation.33 The trial of mesenchymal stem cell augmentation by Lamas et al was terminated early due to safety concerns33 as was the study by Walton et al which assessed the RESTORE patch.34 Study population sizes ranged from 13 to 105 patients for RCTs (age range 29–85 years), and 9 to 152 patients (age range 36–83 years) among observational comparative studies, with a predominance of male participants across all studies. Only two studies included the full spectrum of full thickness tear sizes, with most studies instead restricting recruitment to large or massive tears of the supraspinatus and infraspinatus (table 1). Other eligibility criteria were highly heterogeneous (online supplemental table 1).
Table 1.
Patch type and population demographics
| Study | Patch type | Brand | Surgical approach | Surgical patch technique | Patient demographics | ||||||||
| Xenograft | Human | Synthetic | Age at surgery, mean (range or ±SD) |
Gender, n male (%) | Tear size | ||||||||
| Dermal | Intestinal | Other | Allograft* | Autograft | Resorbable | Non-resorbable | |||||||
| Randomised comparative studies | |||||||||||||
| Avanzi37 | ✓ | Conexa | Arthroscopic | On-lay | 68 (58–78) | 14 (30) | Small to medium† | ||||||
| Control | 66 (54–76) | 22 (48) | Small to medium† | ||||||||||
| Barber36 | ✓ | Graftjacket | Arthroscopic | On-lay | 56 (43–69) | 18 (82) | Small to massive‡ | ||||||
| Control | 56 (34–72) | 13 (65) | Small to large‡ | ||||||||||
| Byrant35 | ✓ | Restore | Open | On-lay | 55 (29–40) | 29 (85) | Small to massive‡ | ||||||
| Control | 58 (40–81) | 22 (79) | Small to massive‡ | ||||||||||
| Cai38 | NR | Collagen matrix | Arthroscopic | On-lay | 62 (50–85) | 24 (47) | Large to massive† | ||||||
| Control | 61 (50–80) | 32 (60) | Large to massive† | ||||||||||
| Ianotti39 | ✓ | Restore | Open | On-lay | 58 (NR) | 11 (73) | Large to massive‡ | ||||||
| Control | 57 (NR) | 12 (80) | Large to massive‡ | ||||||||||
| Lamas33 | ✓§ | MSCs+OrthADAPT | Open | On-lay | 57 (±6.5) | 6 (75) | NR | ||||||
| ✓§ | OrthADAPT | 61 (±3.8) | 2 (40) | ||||||||||
| Leuzinger29 | ✓ | Graftjacket | Arthroscopic | On-lay | 66 (51–81) | 20 (71) | Massive¶ | ||||||
| ✓ | Artelon | 68 (52–79) | 23 (22) | Massive¶ | |||||||||
| ✓ | Restore | 68 (50–82) | 20 (69) | Massive¶ | |||||||||
| Non-randomised comparative studies | |||||||||||||
| Ciampi30 | ✓ | Repol Angimesh | Open | On-lay | 66 (57–77) | 41 (79) | Massive¶ | ||||||
| ✓** | Tutopatch | 66 (58–76) | 38 (78) | Massive¶ | |||||||||
| Control | 67 (58–77) | 35 (69) | Massive¶ | ||||||||||
| Flury41 | ✓ | DX reinforcement matrix + PRP | Arthroscopic | On-lay | 67 (±3.1) | 6 (30) | NR | ||||||
| Control | 65 (±3.3) | 6 (30) | |||||||||||
| Gilot40 | ✓ | Arthroflex | Arthroscopic | On-lay | 58 (±6.2) | 8 (60) | Large to massive‡ | ||||||
| Control | 62 (±4.6) | 7 (47) | Large to massive‡ | ||||||||||
| Ito42 | ✓‡‡ | Fascia lata (cadaveric) | Open | Bridging | 63 (49–70) | 6 (67) | Large to massive‡ | ||||||
| Control | 52 (36–66) | 10 (83) | Large to massive‡ | ||||||||||
| Jeon43 | ✓ | Biceps (long head) | Arthroscopic | Bridging | 62 (46–82) | 14 (45) | Medium‡ | ||||||
| Control | 63 (46–82) | 16 (48) | Medium to large‡ | ||||||||||
| Maillot31 | ✓ | Conexa | Open | On-lay | 56 (46–63) | 5 (45) | Medium to massive‡ | ||||||
| Standard repair | Arthroscopic | 58 (45–71) | 5 (42) | Medium to massive‡ | |||||||||
| Debridement | Arthroscopic | 60 (54–76) | 3 (33) | Medium to massive‡ | |||||||||
| Mori13 | ✓ | Fascia lata | Arthroscopic | Bridging | 65 (±8.9) | 17 (71) | Medium to massive‡ | ||||||
| Control | 65 (±9.2) | 10 (42) | Medium to massive‡ | ||||||||||
| Mori56 | ✓ | Fascia lata + grade 1–2 atrophy | Arthroscopic | Bridging | 65 (±9.0) | 18 (69) | Large to massive‡ | ||||||
| ✓ | Fascia lata + grade 3–4 atrophy | 67 (±6.2) | 11 (58) | Large to massive‡ | |||||||||
| Tempelaere44 | ✓ | Quadriceps tendon | Open | Bridging | NR | 18 (78) | Massive†† | ||||||
| Control | Arthroscopic | NR | 15 (56) | Massive†† | |||||||||
| Veen32 | ✓ | Biceps (long head) | Arthroscopic | Bridging | 64 (61–67) | 3 (75) | Massive¶†† | ||||||
| Control | 65 (57–72) | 1 (3) | Massive¶†† | ||||||||||
| Vitali46 | ✓ | ✓ | Repol Angimesh + biceps (long head) | Open | Bridging | 66 (55–78) | 15 (25) | Massive¶ | |||||
| Control | 67 (56–77) | 18 (30) | Massive¶ | ||||||||||
| Walton34 | ✓ | Restore | Open | On-lay | 60 (±3.5) | 10 (67) | Large to massive† | ||||||
| Control | 59 (±3.1) | 11 (69) | Large to massive† | ||||||||||
| Yoon45 | ✓ | Allocover | Arthroscopic | Bridging | 64 (±8.7) | 9 (43) | Large to massive‡ | ||||||
| Control | 62 (±6.7) | 26 (48) | Large to massive‡ | ||||||||||
| Non-comparative studies | |||||||||||||
| Agrawal57 | ✓ | Allopatch HD | Arthroscopic | On-lay | 54 (47–69) | 10 (71) | Large to massive‡ | ||||||
| Audenaert58 | ✓ | Mersilene | Open | Bridging | 67 (51–80) | 23 (56) | Massive¶ | ||||||
| Badhe59 | ✓ | Zimmer collagen repair patch | Open | Bridging | 66 (46–80) | 5 (50) | Massive‡¶ | ||||||
| Bektaser60 | ✓ | Coracoacromial ligament | Open | On-lay | 54.3 (39–66) | 4 (8.6) | Medium to massive‡ | ||||||
| Bond61 | ✓ | Graftjacket | Arthroscopic | Bridging | 54 (39–74) | 13 (81) | Massive‡¶ | ||||||
| Burkhead62 | ✓ | Graftjacket | Open | On-lay | 56 (NR) | 12 (71) | Massive¶ | ||||||
| Cho63 | ✓ | Permacol | Open | On-lay | 53 (45–57) | 3 (60) | Massive‡¶ | ||||||
| Consigliere64 | ✓ | DX reinforcement matrix | Arthroscopic | On-lay | 74 (65–82) | 6 (40) | Large to massive¶ | ||||||
| Encalada-Diaz65 | ✓ | Polycarbonate polyurethane patch | Open | On-lay | 56 (44–65) | 0 | Small to large‡ | ||||||
| Flury66 | ✓ | Graftjacket or Arthroflex | Arthroscopic | On-lay | 57 (50–68) | 5 (63) | Medium to large‡ | ||||||
| Giannotti67 | ✓ | Zimmer collagen repair patch | Open | Mixed | 66 (50–80) | 4 (44) | Massive† | ||||||
| Gouk68 | ✓ | Graftjacket | Open | Bridging | 54 (44–59) | 6 (86) | Massive‡¶ | ||||||
| Gupta69 | ✓ | Graftjacket | Open | Bridging | 63 (45–83) | 12 (50) | Massive† | ||||||
| Gupta70 | ✓ | Conexa | Open | Bridging | 60 (45–77) | 12 (46) | Massive¶ | ||||||
| Hirooka71 | ✓ | Gore-tex PTFE | Open | Bridging | 62 (44–75) | 20 (74) | Small to massive‡ | ||||||
| Johnson72 | ✓ | Graftjacket | Open | Bridging | 63 (31–77) | NR | Large to massive‡¶ | ||||||
| Lederman73 | ✓ | Conexa | Open | On-lay | 56 (40–69) | NR | Large‡ | ||||||
| Lenart74 | ✓ | X-repair | Open | On-lay | 57 (42–68) | 9 (69) | Massive¶ | ||||||
| Malcarney15 | ✓ | Restore | Open | Mixed | NR | NR | NR | ||||||
| Marberry75 | ✓ | Artelon | Open | On-lay | 65 (45–76) | 5 (29) | Massive¶ | ||||||
| Metcalf76 | ✓ | Restore | Open | On-lay | NR | NR | Massive† | ||||||
| Modi77 | ✓ | Graftjacket | Open | Bridging | 62 (47–72) | 41 (67) | Large to massive‡ | ||||||
| Moore50 | ✓‡‡ | Cadaveric | Open | Bridging | 59 (34–81) | 23 (72) | Massive¶ | ||||||
| Nada78 | ✓ | Dacron | Arthroscopic | Bridging | 66 (55–85) | 14 (67) | Massive‡¶ | ||||||
| Neumann79 | ✓ | Conexa | Open | Bridging | 62 (38–82) | 21 (35) | Massive‡§ | ||||||
| Petrie80 | ✓ | LARS | Open | Bridging | 67 (NR) | 21 (70) | Massive† | ||||||
| Petri81 | ✓ | Arthroflex | Open | On-lay | 57 (26–68) | 11 (85) | Large to massive† | ||||||
| Petricciolo82 | ✓ | SportMesh | Open | On-lay | 61 (51–68) | 8 (80) | Subscapularis tears | ||||||
| Phipatanakul47 | ✓ | Restore | Open | On-lay | 48 (31–62) | 9 (82) | Massive† | ||||||
| Proctor83 | ✓ | X-Repair | Arthroscopic | On-lay | 66 (52–89) | NR | Massive¶ | ||||||
| Rhee84 | ✓ | Biceps (long head) | Mixed | Bridging | 61 (46–79) | 11 (35) | Massive‡¶ | ||||||
| Rotini85 | ✓ | Acellular human dermal matrix | Mixed | On-lay | 48 (37–55) | 5 (100) | Large to massive† | ||||||
| Sano86 | ✓ | Biceps (long head) | Open | Bridging | 64 (48–79) | 12 (86) | Massive¶ | ||||||
| Scheibel48 | ✓ | Periosteum | Open | On-lay | 59 (44–71) | 16 (70) | NR | ||||||
| Schlegel49 | ✓§§ | Collagen sheet | Arthroscopic | On-lay | 54 (34–75) | 19 (58) | N/A—partial thickness | ||||||
| Sclamberg87 | ✓ | Restore | Open | Mixed | 67 (52–79) | 7 (64) | Large to massive‡ | ||||||
| Sears88 | ✓ | Graftjacket | Arthroscopic | On-lay | |||||||||
| ✓ | Tissuemend | 50 (37–70) | NR | NR | |||||||||
| ✓ | Conexa | ||||||||||||
| Smolen89 | ✓ | Pitch-Patch | Arthroscopic | On-lay | 64 (41–75) | 34 (68) | Massive¶ | ||||||
| Venouziou90 | ✓ | Graftjacket | Open | Bridging | 54 (33–64) | 9 (64) | Massive† | ||||||
| Wong91 | ✓ | Graftjacket | Arthroscopic | Bridging | 53 (39–67) | 36 (80) | Massive† | ||||||
Control refers to rotator cuff repair without augmentation. For definitions of ‘On-lay’ and ‘Bridging’ see the Methods section.
*Allograft patches constructed from decellularised human dermis.
†Size of tear as reported by study authors—no details provided on classification used and insufficient detail to enable post hoc classification by review authors (MB and NSN).
‡DeOrio et al17 (J Bone Joint Surg Am 1984:66:563–7).
§Patch derived from equine pericardium.
¶Gerber et al18 (J Bone Joint Surg Am 2000;82:505–15).
**Patch constructed from decellularised bovine pericardium.
††Defined as grade 3 retraction according to Patte classification92 (Clin Orthop Relat Res 1990;254:81–6).
‡‡Cadaveric source of allograft, irradiated but not decellularised.
§§Patch derived from bovine achilles.
DX, dermal xenograft; HD, Human Dermis; LARS, Ligament Augmentation Reconstruction System; MSC, mesenchymal stem cells; NA, Not applicable; NR, not reported; PRP, Platelet-Rich Plasma; PTFE, polytetrafluoroethylene.
bmjopen-2020-039552supp001.pdf (573.3KB, pdf)
Surgical characteristics
Across all the comparative studies a total of 15 different patches was used. Decellularised xenograft patches were the most commonly investigated (n=9; Restore n=4). Surgical techniques could be classified as fully arthroscopic (55%, n=11), open (35%, n=7) or a mixture of both (10%, n=2). Regarding the method of patch utilisation, a larger proportion of studies investigated an ‘on-lay’ (60%, n=12) rather than a ‘bridging’ (40%, n=8) technique.
Risk of bias
Assessment of bias was conducted for all RCTs and comparative studies (online supplemental tables 2 and 3). For the study by Bryant et al35 some concerns over bias were identified but with the remaining RCTs assessed as having a high risk. These findings are based on inadequate randomisation methodologies and a lack of study methodology detail, in particular surrounding blinding of patients and outcome assessors. All observational comparative studies had a serious risk of bias, centring around the potential for confounding, bias in patient selection and outcome measurement.
Outcomes summaries
Shoulder-specific function and pain scores
Eleven different outcomes scores were used to assess shoulder-specific function and pain (online supplemental table 1). The Constant Scale (70%), American Shoulder and Elbow Surgeons (ASES) Score (40%) and University of California Los Angles (UCLA) Scale (30%) were most commonly reported in the comparative studies with most studies reporting multiple functional scores.
Among RCTs, only one study found a sustained, statistically meaningful improvement in ASES and Constant Scores, but not UCLA Scale, 2 years after implantation of an allograft patch (online supplemental table 4).36 A further two studies, investigating a dermal xenograft (DX)37 and an unspecified non-proprietary patch,38 reported initial improvements in Constant Scores at 12 months, but this was not sustained at longer (2 years) follow-up. The two RCTs investigating decellularised porcine small intestine submucosa (Restore)35 39 failed to demonstrate an improvement in patient-reported outcomes at 1–2 years follow-up, while the study by Leuzinger et al29 only undertook intragroup comparisons between preoperative and postoperative Constant Scores, reporting similar improvements following implantation of an allograft, xenograft or synthetic patch.
For the non-randomised comparative studies, only three reported a significant improvement in functional shoulder scores for synthetic,30 human allograft40 and fascia lata autografts.13 The remaining studies found no significant improvement,31 34 41–45 while the studies by Ito et al,42 Veen et al32 and Vitali et al46 did not undertake intergroup comparisons.
Repair failure
Integrity of the surgical repair was assessed by all RCTs and 11 (84%) observational comparative studies, with a re-tear rate ranging from 2% to 100% following patch implantation and 13% to 65% following a standard RCR (online supplemental table 5). MRI was the the most common imaging modality (72%) used to diagnose re-tears, with an MR arthrogram used in a further 17% of studies. The majority of studies (72%) undertook postoperative imaging after 1–2 years but with considerable heterogeneity existing in the radiological classification of re-tears and with seven studies30 32 33 38 40 42 46 not providing any details. While the RCTs investigating human allograft (Graftjacket),36 DX (Conexa)37 and an unspecified collagen patch,38 each demonstrated a significantly lower failure rate in the augmentation arm, neither of the RCTs investigating the small-intestine submucosa xenograft patch (Restore)35 39 found any reduction in re-tear rate. In conflict with these findings, a multipatch comparative study29 found no difference in failure rate between xenograft (Restore) and two different patches; human allograft (Graftjacket) or synthetic (Artelon). Among the observational comparative studies, significantly lower rates of re-tears were reported with augmentation using synthetic (Repol Angimesh),30 autograft (fascia lata)13 or allograft patches (Arthroflex and Allocover),40 45 while no improvement in re-tears was observed following augmentation with DX (reinforcement matrix),41 long head of biceps tendon autograft43 or for the Restore34 patch.
Pain scores
Only two studies (Gilot et al, Athroflex;41 Mori et al, fascia lata13) reported significant reduction in pain when compared against standard repair (online supplemental table 6). Interestingly, the study by Walton et al, who used a ‘mean activity pain score’, found an increase in pain for the first 3 months following implantation of the Restore patch, which subsequently normalised by 6 months.34 The remaining 10 studies either did not report intergroup comparisons (n=5),30 32 33 42 46 or found no significant difference in pain scores between treatment arms (n=5).39 43–45
Quality of life
Only five comparative trials reported the use of either the SF-12, SF-36 or EQ-5D Scores (online supplemental table 7). When compared with standard repair, two RCTs investigating porcine SIS xenograft (Restore) found no difference in the physical or mental components of SF-36.35 39 Similarly, an RCT (Avanzi et al)37 and an observational comparative study (Flury et al)41 assessing different DXs did not find an improved EQ-5D at 2-year follow-up. Conversely, a comparative study using human allograft (Athroflex) reported a significant improvement in all components of SF-12 at 6 months and 2 years postoperatively (though not at 3 months).40
Complications
Forty-nine studies provided data on complications, of which 24 studies reported the occurrence of 83 complications in a total population of 1567 patients undergoing any form of augmentative surgery and 488 patients receiving a standard RCR (online supplemental table 5). The overall crude complications rates were 4.5% for patients undergoing any form of patch augmentation and 1.6% following non-augmentative surgery. However, by excluding six studies in the augmentation group which had particularly high rates of complications (20%–74%) following quadriceps tendon, MSC seeded xenograft, Restore patch or humeral periosteal augmented repair,33 34 39 44 47 48 the overall rate of complications following patch augmentation was 2.6%. An inflammatory response was recorded in 18 patients (all reported complications are detailed in online supplemental table 5). The majority of these events (n=11) occurred in patients who received an SIS xenograft (Restore) patch, but with reactions also reported after implantation of bovine-derived,49 equine-derived,33 irradiated50 or decellularised29 human allograft and synthetic patches.29 Excluding all adverse events concerning the Restore patch, which has been withdrawn from the marketplace, the crude complication rate for patches in current clinical use was 2.1%.
Meta-analysis
Shoulder-specific function and pain
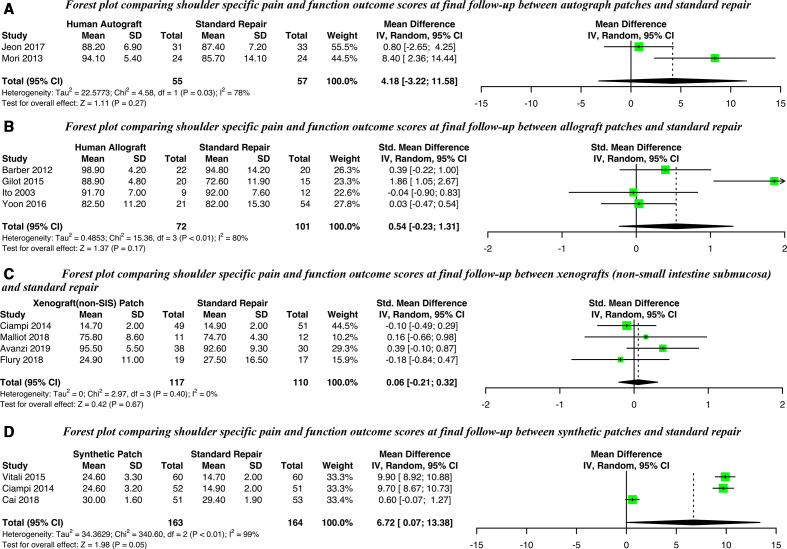
Of the 20 comparative studies, 12 provided sufficient data on postoperative functional outcome scores to be included in the meta-analysis (figure 2). An improvement on the UCLA shoulder scale was observed for synthetic patches at long-term (range 24–36 months) follow-up (mean difference 6.72, 95% CI 0.07 to 13.38) but not in the ASES Score of studies of autografts (mean difference 4.18, 95% CI −3.22 to 11.58). Studies of allografts or xenografts derived from dermis or pericardium (non-SIS) used differing measures but with no significant standardised mean differences observed for either. Level of heterogeneity, across all patch types, was generally very high (I2 >70%). Insufficient data were available for xenografts derived from intestinal submucosa (SIS). Sensitivity analyses did not find any impact of study design (randomised vs observational) except for synthetic patches where the sole RCT differed from the observational studies (online supplemental figure 1).
Figure 2.
Forest plots comparing shoulder-specific functional outcomes scores at final follow-up for (A) Autografts, (B) Allografts, (C) Xenografts (non-SIS) or (D) Synthetic patches against standard repair alone. SIS, small intestine submucosa; IV, Random, a random-effects meta-analysis is applied, with weights based on inverse variances.
bmjopen-2020-039552supp002.pdf (1MB, pdf)
Re-tear rate
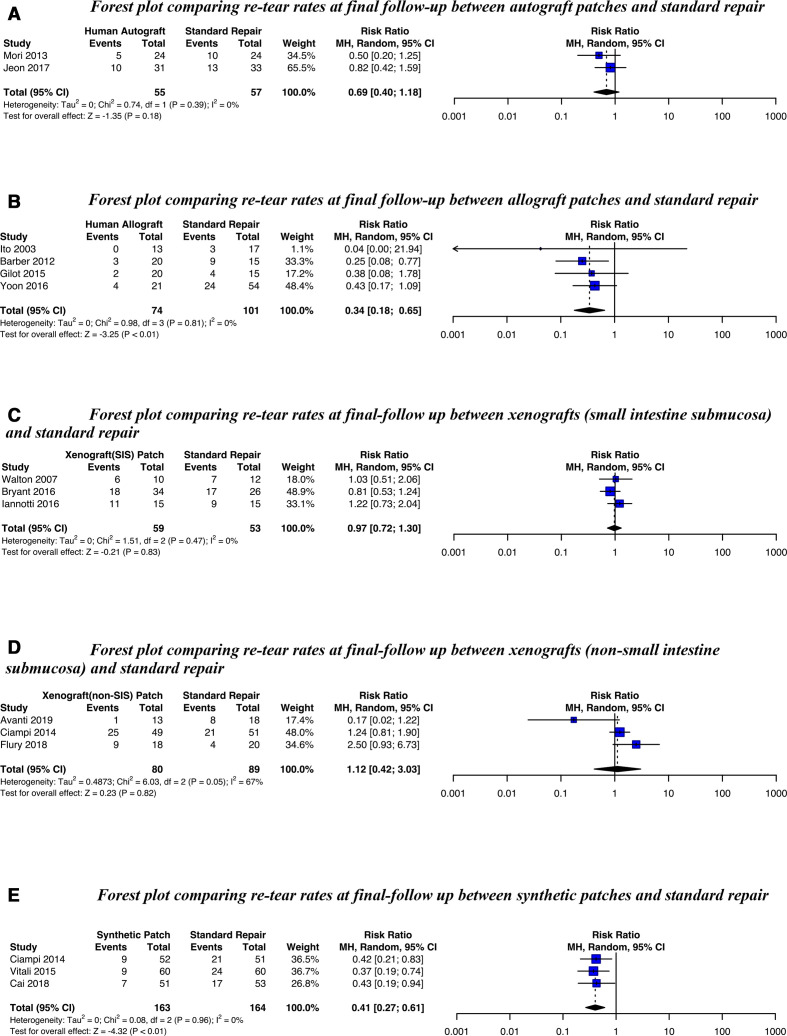
Fourteen studies were included in a meta-analysis for re-tear rate (figure 3). A significantly lower re-tear rate was seen for allograft patches (RR 0.34, 95% CI 0.18 to 0.65) and synthetic patches (RR 0.41, 95% CI 0.27 to 0.61) but not for autografts, SIS-derived or non-SIS-derived xenografts (there was substantial heterogeneity for the latter, I2=67%). Meta-analysis results were not influenced by study design (online supplemental figure 2).
Figure 3.
Forest plots comparing re-tear rates at final follow-up for (A) Autografts, (B) Allografts, (C) Xenografts (SIS) (D) Xenografts (non-SIS) or (E) Synthetic patches against standard repair alone. SIS, small intestine submucosa; MH, Random, a random-effects meta-analysis is applied, with weights based on the Mantel-Haenszel method.
Pain
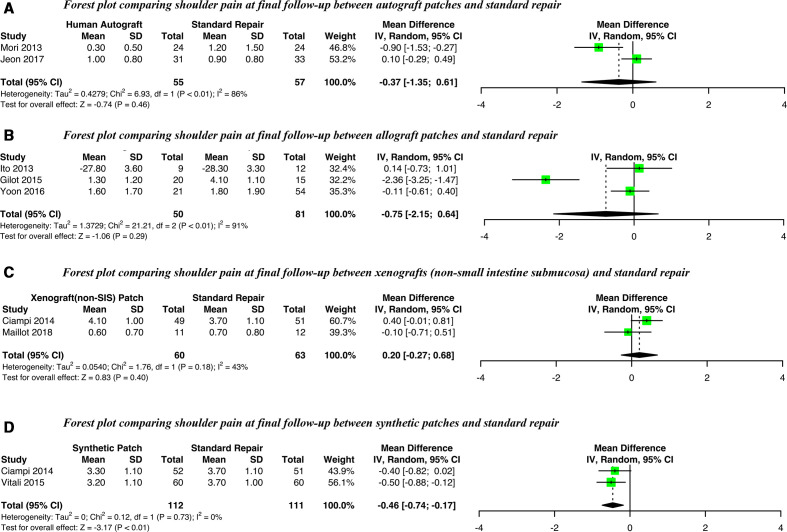
Eight observational comparative studies had sufficient data for a meta-analysis of postoperative pain (figure 4). A small, possibly non-clinically significant,51 improvement in postoperative pain was only observed for synthetic patches (mean difference −0.46, 95% CI −0.74 to −0.17, I2=0%). Meta-analyses of allograft, autograph and xenograph (non-SIS) patches did not show any statistically significant differences. Level of heterogeneity was generally very high (I2 >70%). Insufficient data were available for a meta-analysis of postoperative pain following augmentation with xenograft patches derived from SIS.
Figure 4.
Forest plots comparing postoperative pain at final follow-up for (A) Autografts, (B) Allografts, (C) Xenografts (non-SIS) or (D) Synthetic patches against standard repair alone. SIS, small intestine submucosa; IV, Random, a random-effects meta-analysis is applied, with weights based on inverse variances.
Health-related quality of life
There were insufficient data available to meta-analyse the effect of patch augmentation on quality of life.
Discussion
The use of medical implants has recently come under increasing scrutiny. Surgical repair of the rotator cuff with patch augmentation has been proposed as a method of improving rates of tendon healing and patient outcomes. This systematic review is the largest and most comprehensive systematic appraisal of the clinical effectiveness and safety of such implants to date. Overall, the current evidence is not sufficiently robust to determine the effectiveness of patch-augmented RCR compared with standard repair alone. While this meta-analysis suggests a small improvement in pain and patient reported outcome measures (PROMS) for synthetic patches, and a moderate reduction in re-tear rate for synthetic and human allograft patches, study bias and heterogeneity mean that these results need to be interpreted very cautiously. Further, it is unclear if the observed 6-point improvement in UCLA Score for synthetic patches is clinically meaningful. To date, the minimal clinically importance difference for UCLA shoulder score following RCR has not been established.52 However, a threshold of 30 UCLA points after 2 years has been proposed as an absolute cut-off signifying treatment success following RCR.53 In the studies investigating synthetic polypropylene patches, augmentation failed to meet this threshold.30 46 Similarly, the 0.46-point reduction in VAS pain scores is unlikely to be clinically meaningful.51
Across 49 studies reporting complications (adverse events) with a combined population of 2055 participants, the crude complication rate was marginally higher for augmented (2.1%) than standard (1.6%) repairs, with specific safety concerns associated with certain patches (Restore)39 or techniques (Quadriceps allograft, Humeral Periosteal allograft, MSCs embedded in decellularised bovine pericardium).33 44 48
Strengths and limitations of the study
Strengths of this this review include an a priori published protocol;16 a comprehensive search strategy; inclusion of non-English language articles and duplicate assessment of eligibility, risk of bias and data extraction. Nonetheless, there remain several limitations to the current review, which are mainly a reflection of the quality of published primary research available. Only seven RCTs have been published, of which three refer to a product (Restore) that has now been withdrawn from the market due to safety concerns,29 36 39 and the study by Lamas et al was terminated due to excessive adverse events.33 Second, substantial heterogeneity between studies was observed with the majority of studies also judged to have a high risk of bias, seriously limiting our ability to draw firm recommendations. An exhaustive exploration of the heterogeneity has not been undertaken and indeed such an analysis was not declared a priori in our protocol paper.16 However, separating studies by patch type did influence the degree of heterogeneity and we would therefore recommend patch type should be considered in the design of future reviews. Finally, it should be noted that given the limited number of studies for the different patch types and the relatively small typical size of studies, the observed effects identified could reflect, to a degree, chance findings. With this in mind, the observed differences in outcomes between patch types should be interpreted cautiously and warrant confirmation by further trials.
In comparison with previous systematic reviews,19 22 23 we have included four additional RCTs29 33 37 38 and five observational comparative studies,32 41–43 45 representing 324 patients, not otherwise identified. Results from our meta-analysis are, in part, consistent with a previous analysis which found an overall reduction in re-tear rate and improved ASES Scores following patch augmentation.22 The substantial number of additional studies included in this current review provide greater precision and, while a subgroup analysis was not originally specified in our protocol, they have allowed us to hypothesise that patch type may have an effect on patient outcomes. The occurrence of adverse events with only certain patch types adds some credibility to this notion. Previous reviews of augmented RCR have, on the basis of a presumed effect on patient outcome, excluded studies based on the size of rotator cuff tear or surgical technique (on-lay or bridging).19 It is possible that each technique reflects different patient cohorts, for example, the use of bridging scaffolds may represent larger, more chronic or even recurrent rotator cuff tears. However, we were unable to detect any overall difference in patient-reported outcomes, re-tear rate or pain scores between studies reporting on-lay or bridging techniques. It should be noted that differences in terminology makes comparison of these surgical techniques challenging—many studies use the terms irreparable, bridging, interposition or reconstruction interchangeably. To help facilitate the future interrogation of the relationship between surgical technique and outcomes we would suggest that only the terms on-lay augmentation or bridging reconstruction be used in accordance with previously published definitions.19 Similarly, a frequent lack of detail on how tear size was determined impedes any examination of tear size on treatment effectiveness. Trials should clearly describe, in a reproducible way, the classification system used when determining rotator cuff tear size.
There are a growing number of patches available for the augmentation of RCR. Despite the safety-related withdrawal of certain patches,34 as well as wider concerns surrounding medical device54 and mesh implantation,54 rigorous clinical evaluation of patch augmentation is lacking. We were particularly concerned by the absence of publicly available research for several patches currently in clinical use (eg, d-cell and Leeds-Kuff). While some studies have indicated promise for specific patches, firm recommendations in terms of patch type or surgical technique cannot be made at present. There remains a need for well-designed comparative (preferably multicentre RCTs) studies that are capable of robustly evaluating the effectiveness and safety of multiple patch types. Furthermore, routine reporting of registry data patch could address the current lack of robust safety data for cuff-augmented rotated cuff repair.55
Supplementary Material
Footnotes
Twitter: @mathewbaldwin3, @ProfJACook
Contributors: JAC and AJC conceived the idea, and JAC lead the project. JLR, AR, DB, MD and SH contributed to study design and securing funding. NM contributed to the management of the study. Article screening, data extraction, analysis and interpretation undertaken by MB, NSN and JAC. Literature searches undertaken by GG and updates by GG, MB and NSN. All authors contributed to manuscript writing while MB and JAC undertook reviewer requested revisions.
Funding: The study was funded by the National Institute for Health Research (NIHR) Health Technology Assessment Programme (HTA 15/103/03), and it was supported by the NIHR-funded Oxford Biomedical Research Centre (BRC).
Disclaimer: The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Competing interests: AC has applied for a patent for a device which would be eligible for this type of review if it is approved for clinical use, AR has received educational and research grants from DePuy Ltd.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. The data set used and analysed for this review will be available from the corresponding author upon a reasonable request.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Linsell L, Dawson J, Zondervan K, et al. . Prevalence and incidence of adults consulting for shoulder conditions in UK primary care; patterns of diagnosis and referral. Rheumatology 2006;45:215–21. 10.1093/rheumatology/kei139 [DOI] [PubMed] [Google Scholar]
- 2.van der Windt DA, Koes BW, Boeke AJ, et al. . Shoulder disorders in general practice: prognostic indicators of outcome. Br J Gen Pract 1996;46:519–23. [PMC free article] [PubMed] [Google Scholar]
- 3.Virta L, Joranger P, Brox JI, et al. . Costs of shoulder pain and resource use in primary health care: a cost-of-illness study in Sweden. BMC Musculoskelet Disord 2012;13:17 10.1186/1471-2474-13-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy RJ, Carr AJ. Shoulder pain. BMJ Clin Evid 2010;2010:1107. [PMC free article] [PubMed] [Google Scholar]
- 5.Sher JS, Uribe JW, Posada A, et al. . Abnormal findings on magnetic resonance images of asymptomatic shoulders. J Bone Joint Surg Am 1995;77:10–15. 10.2106/00004623-199501000-00002 [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto A, Takagishi K, Osawa T, et al. . Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg 2010;19:116–20. 10.1016/j.jse.2009.04.006 [DOI] [PubMed] [Google Scholar]
- 7.Carr A, Cooper C, Campbell MK, et al. . Effectiveness of open and arthroscopic rotator cuff repair (UKUFF). Bone Joint J 2017;99-B:107–15. 10.1302/0301-620X.99B1.BJJ-2016-0424.R1 [DOI] [PubMed] [Google Scholar]
- 8.Judge A, Murphy RJ, Maxwell R, et al. . Temporal trends and geographical variation in the use of subacromial decompression and rotator cuff repair of the shoulder in England. Bone Joint J 2014;96-B:70–4. 10.1302/0301-620X.96B1.32556 [DOI] [PubMed] [Google Scholar]
- 9.Rashid MS, Cooper C, Cook J, et al. . Increasing age and tear size reduce rotator cuff repair healing rate at 1 year. Acta Orthop 2017;88:606–11. 10.1080/17453674.2017.1370844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozaki J, Fujimoto S, Masuhara K, et al. . Reconstruction of chronic massive rotator cuff tears with synthetic materials. Clin Orthop Relat Res 1986;202:173–83. 10.1097/00003086-198601000-00022 [DOI] [PubMed] [Google Scholar]
- 11.Shea KP, Obopilwe E, Sperling JW, et al. . A biomechanical analysis of gap formation and failure mechanics of a xenograft-reinforced rotator cuff repair in a cadaveric model. J Shoulder Elbow Surg 2012;21:1072–9. 10.1016/j.jse.2011.07.024 [DOI] [PubMed] [Google Scholar]
- 12.Shea KP, McCarthy MB, Ledgard F, et al. . Human tendon cell response to 7 commercially available extracellular matrix materials: an in vitro study. Arthroscopy 2010;26:1181–8. 10.1016/j.arthro.2010.01.020 [DOI] [PubMed] [Google Scholar]
- 13.Mori D, Funakoshi N, Yamashita F. Arthroscopic surgery of irreparable large or massive rotator cuff tears with low-grade fatty degeneration of the infraspinatus: patch autograft procedure versus partial repair procedure. Arthroscopy 2013;29:1911–21. 10.1016/j.arthro.2013.08.032 [DOI] [PubMed] [Google Scholar]
- 14.Sedrakyan A, Campbell B, Merino JG, et al. . IDEAL-D: a rational framework for evaluating and regulating the use of medical devices. BMJ 2016;353:i2372–7. 10.1136/bmj.i2372 [DOI] [PubMed] [Google Scholar]
- 15.Malcarney HL, Bonar F, Murrell GAC. Early inflammatory reaction after rotator cuff repair with a porcine small intestine submucosal implant. Am J Sports Med 2005;33:907–11. 10.1177/0363546504271500 [DOI] [PubMed] [Google Scholar]
- 16.Greenall G, Carr A, Beard D, et al. . Systematic review of the surgical management of rotator cuff repair with an augmentative patch: a feasibility study protocol. Syst Rev 2018;7:187 10.1186/s13643-018-0851-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeOrio JK, Cofield RH. Results of a second attempt at surgical repair of a failed initial Rotator-cuff repair. The Journal of Bone & Joint Surgery 1984;66:563–7. 10.2106/00004623-198466040-00011 [DOI] [PubMed] [Google Scholar]
- 18.Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am 2000;82:505–15. 10.2106/00004623-200004000-00006 [DOI] [PubMed] [Google Scholar]
- 19.Ferguson DP, Lewington MR, Smith TD, et al. . Graft utilization in the augmentation of Large-to-Massive rotator cuff repairs. Am J Sports Med 2016;44:2984–92. 10.1177/0363546515624463 [DOI] [PubMed] [Google Scholar]
- 20.Coghlan JA, Buchbinder R, Green S, et al. . Surgery for rotator cuff disease. Cochrane Database Syst Rev 2008:CD005619. 10.1002/14651858.CD005619.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thangarajah T, Pendegrass CJ, Shahbazi S, et al. . Augmentation of rotator cuff repair with soft tissue scaffolds. Orthop J Sports Med 2015;3:232596711558749 10.1177/2325967115587495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey JR, Kim C, Alentorn-Geli E, et al. . Rotator cuff matrix augmentation and interposition: a systematic review and meta-analysis. Am J Sports Med 2018;036354651877476. [DOI] [PubMed] [Google Scholar]
- 23.Steinhaus ME, Makhni EC, Cole BJ, et al. . Outcomes after patch use in rotator cuff repair. Arthroscopy 2016;32:1676–90. 10.1016/j.arthro.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 24.Papalia R, Franceschi F, Zampogna B, et al. . Augmentation techniques for rotator cuff repair. Br Med Bull 2013;105:107–38. 10.1093/bmb/lds029 [DOI] [PubMed] [Google Scholar]
- 25.Higgins JPT, Altman DG, Gøtzsche PC, et al. . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sterne JAC, Hernán MA, Reeves BC, et al. . ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shamseer L, Moher D, Clarke M, et al. . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;349:g7647–25. 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 28.Cook JA, Merritt N, Rees JL, et al. . Patch-augmented rotator cuff surgery (PARCS) study—protocol for a feasibility study. Pilot Feasibility Stud 2018;4:188 10.1186/s40814-018-0380-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leuzinger J, Sternberg C, Smolen D, et al. . [Patch Augmentation in Rotator Cuff Repair Surgery with Elder Patients]. Z Orthop Unfall 2016;154:504–12. 10.1055/s-0042-106475 [DOI] [PubMed] [Google Scholar]
- 30.Ciampi P, Scotti C, Nonis A, et al. . The benefit of synthetic versus biological patch augmentation in the repair of posterosuperior massive rotator cuff tears. Am J Sports Med 2014;42:1169–75. 10.1177/0363546514525592 [DOI] [PubMed] [Google Scholar]
- 31.Maillot C, Harly E, Demezon H, et al. . Surgical repair of large-to-massive rotator cuff tears seems to be a better option than patch augmentation or débridement and biceps tenotomy: a prospective comparative study. J Shoulder Elbow Surg 2018;27:1545–52. 10.1016/j.jse.2018.05.023 [DOI] [PubMed] [Google Scholar]
- 32.Veen EJD, Diercks RL, Landman EBM, et al. . The results of using a tendon autograft as a new rotator cable for patients with a massive rotator cuff tear: a technical note and comparative outcome analysis. J Orthop Surg Res 2020;15:1–7. 10.1186/s13018-020-1568-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamas JR, García-Fernández C, Tornero-Esteban P, et al. . Adverse effects of xenogenic scaffolding in the context of a randomized double-blind placebo-controlled study for repairing full-thickness rotator cuff tears. Trials 2019;20:1–9. 10.1186/s13063-019-3504-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walton JR, Bowman NK, Khatib Y, et al. . Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am 2007;89:786–91. 10.2106/JBJS.F.00315 [DOI] [PubMed] [Google Scholar]
- 35.Bryant D, Holtby R, Willits K, et al. . A randomized clinical trial to compare the effectiveness of rotator cuff repair with or without augmentation using porcine small intestine submucosa for patients with moderate to large rotator cuff tears: a pilot study. J Shoulder Elbow Surg 2016;25:1623–33. 10.1016/j.jse.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 36.Barber FA, Burns JP, Deutsch A, et al. . A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy 2012;28:8–15. 10.1016/j.arthro.2011.06.038 [DOI] [PubMed] [Google Scholar]
- 37.Avanzi P, Giudici LD, Capone A, et al. . Prospective randomized controlled trial for patch augmentation in rotator cuff repair: 24-month outcomes. J Shoulder Elbow Surg 2019;28:1918–27. 10.1016/j.jse.2019.05.043 [DOI] [PubMed] [Google Scholar]
- 38.Cai Y-Z, Zhang C, Jin R-L, et al. . Arthroscopic rotator cuff repair with graft augmentation of 3-dimensional biological collagen for moderate to large tears: a randomized controlled study. Am J Sports Med 2018;46:1424–31. 10.1177/0363546518756978 [DOI] [PubMed] [Google Scholar]
- 39.Iannotti JP, Codsi MJ, Kwon YW, et al. . Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. J Bone Joint Surg Am 2006;88:1238–44. 10.2106/00004623-200606000-00010 [DOI] [PubMed] [Google Scholar]
- 40.Gilot GJ, Alvarez-Pinzon AM, Barcksdale L, et al. . Outcome of large to massive rotator cuff tears repaired with and without extracellular matrix augmentation: a prospective comparative study. Arthroscopy 2015;31:1459–65. 10.1016/j.arthro.2015.02.032 [DOI] [PubMed] [Google Scholar]
- 41.Flury M, Rickenbacher D, Jung C, et al. . Porcine Dermis Patch Augmentation of Supraspinatus Tendon Repairs: A Pilot Study Assessing Tendon Integrity and Shoulder Function 2 Years After Arthroscopic Repair in Patients Aged 60 Years or Older. Arthroscopy 2018;34:24–37. 10.1016/j.arthro.2017.06.024 [DOI] [PubMed] [Google Scholar]
- 42.Ito J, Morioka T. Surgical treatment for large and massive tears of the rotator cuff. Int Orthop 2003;27:228–31. 10.1007/s00264-003-0459-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeon YS, Lee J, Kim RG, et al. . Does additional biceps augmentation improve rotator cuff healing and clinical outcomes in anterior L-shaped rotator cuff tears? clinical comparisons with arthroscopic partial repair. Am J Sports Med 2017;45:2982–8. 10.1177/0363546517720198 [DOI] [PubMed] [Google Scholar]
- 44.Tempelaere C, Desmoineaux P, Lespagnol F, et al. . Surgical repair of massive rotator cuff tendon tears: autologous quadriceps tendon graft versus arthroscopic repair. Orthop Traumatol Surg Res 2017;103:435–40. 10.1016/j.otsr.2016.12.020 [DOI] [PubMed] [Google Scholar]
- 45.Yoon JP, Chung SW, Kim JY, et al. . Outcomes of combined bone marrow stimulation and patch augmentation for massive rotator cuff tears. Am J Sports Med 2016;44:963–71. 10.1177/0363546515625044 [DOI] [PubMed] [Google Scholar]
- 46.Vitali M, Cusumano A, Pedretti A, et al. . Employment of synthetic patch with augmentation of the long head of the biceps tendon in irreparable lesions of the rotator cuff. Tech Hand Up Extrem Surg 2015;19:32–9. 10.1097/BTH.0000000000000072 [DOI] [PubMed] [Google Scholar]
- 47.Phipatanakul WP, Petersen SA. Porcine small intestine submucosa xenograft augmentation in repair of massive rotator cuff tears. Am J Orthop 2009;38:572–5. [PubMed] [Google Scholar]
- 48.Scheibel M, Brown A, Woertler K, et al. . Preliminary results after rotator cuff reconstruction augmented with an autologous periosteal flap. Knee Surg Sports Traumatol Arthrosc 2007;15:305–14. 10.1007/s00167-006-0173-z [DOI] [PubMed] [Google Scholar]
- 49.Schlegel TF, Abrams JS, Bushnell BD, et al. . Radiologic and clinical evaluation of a bioabsorbable collagen implant to treat partial-thickness tears: a prospective multicenter study. J Shoulder Elbow Surg 2018;27:242–51. 10.1016/j.jse.2017.08.023 [DOI] [PubMed] [Google Scholar]
- 50.Moore DR, Cain EL, Schwartz ML, et al. . Allograft reconstruction for massive, irreparable rotator cuff tears. Am J Sports Med 2006;34:392–6. 10.1177/0363546505281237 [DOI] [PubMed] [Google Scholar]
- 51.Tashjian RZ, Deloach J, Porucznik CA, et al. . Minimal clinically important differences (MCID) and patient acceptable symptomatic state (pass) for visual analog scales (vas) measuring pain in patients treated for rotator cuff disease. J Shoulder Elbow Surg 2009;18:927–32. 10.1016/j.jse.2009.03.021 [DOI] [PubMed] [Google Scholar]
- 52.Wylie JD, et al. Functional outcomes assessment in shoulder surgery. World J Orthop 2014;5:623–33. 10.5312/wjo.v5.i5.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu S, Chen JY, Lie HME, et al. . Determination of threshold scores for treatment success after arthroscopic rotator cuff repair using Oxford, constant, and University of California, Los Angeles shoulder scores. Arthroscopy 2019;35:304–11. 10.1016/j.arthro.2018.07.047 [DOI] [PubMed] [Google Scholar]
- 54.Berry MG, Stanek JJ. The PIP mammary prosthesis: a product recall study. J Plast Reconstr Aesthet Surg 2012;65:697–704. 10.1016/j.bjps.2012.02.019 [DOI] [PubMed] [Google Scholar]
- 55.Hirst A, Philippou Y, Blazeby J, et al. . No surgical innovation without evaluation: evolution and further development of the ideal framework and recommendations. Ann Surg 2019;269:211–20. 10.1097/SLA.0000000000002794 [DOI] [PubMed] [Google Scholar]
- 56.Mori D, Funakoshi N, Yamashita F, et al. . Effect of fatty degeneration of the infraspinatus on the efficacy of arthroscopic patch autograft procedure for large to massive rotator cuff tears. Am J Sports Med 2015;43:1108–17. 10.1177/0363546515569680 [DOI] [PubMed] [Google Scholar]
- 57.Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg 2012;6:36–44. 10.4103/0973-6042.96992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Audenaert E, Van Nuffel J, Schepens A, et al. . Reconstruction of massive rotator cuff lesions with a synthetic interposition graft: a prospective study of 41 patients. Knee Surg Sports Traumatol Arthrosc 2006;14:360–4. 10.1007/s00167-005-0689-7 [DOI] [PubMed] [Google Scholar]
- 59.Badhe SP, Lawrence TM, Smith FD, et al. . An assessment of porcine dermal xenograft as an augmentation graft in the treatment of extensive rotator cuff tears. J Shoulder Elbow Surg 2008;17:S35–9. 10.1016/j.jse.2007.08.005 [DOI] [PubMed] [Google Scholar]
- 60.Bektaser B, et al. Free coracoacromial ligament graft for augmentation of massive rotator cuff tears treated with mini-open repair. Acta Orthop Traumatol Turc 2010;44:426–30. 10.3944/AOTT.2010.2423 [DOI] [PubMed] [Google Scholar]
- 61.Bond JL, Dopirak RM, Higgins J, et al. . Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy 2008;24:403.e1–403.e8. 10.1016/j.arthro.2007.07.033 [DOI] [PubMed] [Google Scholar]
- 62.Burkhead WZ, Schiffern SC, Krishnan SG. Use of graft jacket as an augmentation for massive rotator cuff tears. Semin Arthroplasty 2007;18:11–18. 10.1053/j.sart.2006.11.017 [DOI] [Google Scholar]
- 63.Cho C-H, Lee S-M, Lee Y-K, et al. . Mini-open suture bridge repair with porcine dermal patch augmentation for massive rotator cuff tear: surgical technique and preliminary results. Clin Orthop Surg 2014;6:329–35. 10.4055/cios.2014.6.3.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Consigliere P, Polyzois I, Sarkhel T, et al. . Preliminary Results of a Consecutive Series of Large & Massive Rotator Cuff Tears Treated with Arthroscopic Rotator Cuff Repairs Augmented with Extracellular Matrix. Arch Bone Jt Surg 2017;5:14–21. [PMC free article] [PubMed] [Google Scholar]
- 65.Encalada-Diaz I, Cole BJ, Macgillivray JD, et al. . Rotator cuff repair augmentation using a novel polycarbonate polyurethane patch: preliminary results at 12 months' follow-up. J Shoulder Elbow Surg 2011;20:788–94. 10.1016/j.jse.2010.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flury M. [Arthroscopic rotator cuff repair with patch augmentation]. Oper Orthop Traumatol 2012;24:486–94. 10.1007/s00064-012-0158-7 [DOI] [PubMed] [Google Scholar]
- 67.Giannotti S, Ghilardi M, Dell'osso G, et al. . Study of the porcine dermal collagen repair patch in morpho-functional recovery of the rotator cuff after minimum follow-up of 2.5 years. Surg Technol Int 2014;24:348–52. [PubMed] [Google Scholar]
- 68.Gouk CJC, Shulman RM, Buchan C, et al. . Failure of dermal allograft repair of massive rotator cuff tears in magnetic resonance imaging and clinical assessment. Clin Orthop Surg 2019;11:200–7. 10.4055/cios.2019.11.2.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gupta AK, Hug K, Berkoff DJ, et al. . Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med 2012;40:141–7. 10.1177/0363546511422795 [DOI] [PubMed] [Google Scholar]
- 70.Gupta AK, Hug K, Boggess B, et al. . Massive or 2-tendon rotator cuff tears in active patients with minimal glenohumeral arthritis: clinical and radiographic outcomes of reconstruction using dermal tissue matrix xenograft. Am J Sports Med 2013;41:872–9. 10.1177/0363546512475204 [DOI] [PubMed] [Google Scholar]
- 71.Hirooka A, Yoneda M, Wakaitani S, et al. . Augmentation with a Gore-Tex patch for repair of large rotator cuff tears that cannot be sutured. Journal of Orthopaedic Science 2002;7:451–6. 10.1007/s007760200078 [DOI] [PubMed] [Google Scholar]
- 72.Johnson SM, Cherry J, Thomas N, et al. . Clinical outcomes and ultrasonographic viability of GraftJacket® augmented rotator cuff repair: a prospective follow-up study with mean follow-up of forty-one months. J Clin Orthop Trauma 2019:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lederman ES, Toth AP, Nicholson GP, et al. . A prospective, multicenter study to evaluate clinical and radiographic outcomes in primary rotator cuff repair reinforced with a xenograft dermal matrix. J Shoulder Elbow Surg 2016;25:1961–70. 10.1016/j.jse.2016.02.029 [DOI] [PubMed] [Google Scholar]
- 74.Lenart BA, Martens KA, Kearns KA, et al. . Treatment of massive and recurrent rotator cuff tears augmented with a poly-l-lactide graft, a preliminary study. J Shoulder Elbow Surg 2015;24:915–21. 10.1016/j.jse.2014.09.044 [DOI] [PubMed] [Google Scholar]
- 75.Marberry TA. A synthetic reinforcement patch in repair of challenging two-tendon rotator cuff tears. Shoulder Elbow 2013;5:24–9. 10.1111/j.1758-5740.2012.00210.x [DOI] [Google Scholar]
- 76.Metcalf MH, Savoie FH, Kellum B. Surgical technique for xenograft (SIS) augmentation of Rotator-cuff repairs. Oper Tech Orthop 2002;12:204–8. 10.1053/otor.2002.36298 [DOI] [Google Scholar]
- 77.Modi A, Singh HP, Pandey R, et al. . Management of irreparable rotator cuff tears with the Graftjacket allograft as an interpositional graft. Shoulder & Elbow 2013;5:188–94. 10.1111/sae.12021 [DOI] [Google Scholar]
- 78.Nada AN, Debnath UK, Robinson DA, et al. . Treatment of massive Rotator-cuff tears with a polyester ligament (Dacron) augmentation. J Bone Joint Surg Br 2010;92-B:1397–402. 10.1302/0301-620X.92B10.24299 [DOI] [PubMed] [Google Scholar]
- 79.Neumann JA, Zgonis MH, Rickert KD, et al. . Interposition dermal matrix xenografts: a successful alternative to traditional treatment of massive rotator cuff tears. Am J Sports Med 2017;45:1261–8. 10.1177/0363546516683945 [DOI] [PubMed] [Google Scholar]
- 80.Petrie MJ, Ismaiel AH. Treatment of massive Rotator-cuff tears with a polyester ligament (LARS) patch. Acta Orthop Belg 2013;79:620–5. [PubMed] [Google Scholar]
- 81.Petri M, Warth RJ, Horan MP, et al. . Outcomes after open revision repair of massive rotator cuff tears with biologic patch augmentation. Arthroscopy 2016;32:1752–60. 10.1016/j.arthro.2016.01.037 [DOI] [PubMed] [Google Scholar]
- 82.Petriccioli D, Bertone C, Marchi G, et al. . Open repair of isolated traumatic subscapularis tendon tears with a synthetic soft tissue reinforcement. Musculoskelet Surg 2013;97 Suppl 1:63–8. 10.1007/s12306-013-0260-5 [DOI] [PubMed] [Google Scholar]
- 83.Proctor CS. Long-Term successful arthroscopic repair of large and massive rotator cuff tears with a functional and degradable reinforcement device. J Shoulder Elbow Surg 2014;23:1508–13. 10.1016/j.jse.2014.01.010 [DOI] [PubMed] [Google Scholar]
- 84.Rhee YG, Cho NS, Lim CT, et al. . Bridging the gap in immobile massive rotator cuff tears: augmentation using the tenotomized biceps. Am J Sports Med 2008;36:1511–8. 10.1177/0363546508316020 [DOI] [PubMed] [Google Scholar]
- 85.Rotini R, Marinelli A, Guerra E, et al. . Human dermal matrix scaffold augmentation for large and massive rotator cuff repairs: preliminary clinical and MRI results at 1-year follow-up. Musculoskelet Surg 2011;95:13–23. 10.1007/s12306-011-0141-8 [DOI] [PubMed] [Google Scholar]
- 86.Sano H, Mineta M, Kita A, et al. . Tendon patch grafting using the long head of the biceps for irreparable massive rotator cuff tears. J Orthop Sci 2010;15:310–6. 10.1007/s00776-010-1453-5 [DOI] [PubMed] [Google Scholar]
- 87.Sclamberg SG, Tibone JE, Itamura JM, et al. . Six-Month magnetic resonance imaging follow-up of large and massive rotator cuff repairs reinforced with porcine small intestinal submucosa. J Shoulder Elbow Surg 2004;13:538–41. 10.1016/j.jse.2004.03.005 [DOI] [PubMed] [Google Scholar]
- 88.Sears BW, Choo A, Yu A, et al. . Clinical outcomes in patients undergoing revision rotator cuff repair with extracellular matrix augmentation. Orthopedics 2015;38:e292–6. 10.3928/01477447-20150402-57 [DOI] [PubMed] [Google Scholar]
- 89.Smolen D, Haffner N, Mittermayr R, et al. . Application of a new polyester patch in arthroscopic massive rotator cuff repair-a prospective cohort study. J Shoulder Elbow Surg 2020;29:e11–21. 10.1016/j.jse.2019.05.015 [DOI] [PubMed] [Google Scholar]
- 90.Venouziou AI, Kokkalis ZT, Sotereanos DG. Human dermal allograft interposition for the reconstruction of massive irreparable rotator cuff tears. Am J Orthop 2013;42:63–70. [PubMed] [Google Scholar]
- 91.Wong I, Burns J, Snyder S. Arthroscopic GraftJacket repair of rotator cuff tears. J Shoulder Elbow Surg 2010;19:104–9. 10.1016/j.jse.2009.12.017 [DOI] [PubMed] [Google Scholar]
- 92.Patte D. Classification of rotator cuff lesions. Clin Orthop Relat Res 1990;254:81–6. 10.1097/00003086-199005000-00012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-039552supp001.pdf (573.3KB, pdf)
bmjopen-2020-039552supp002.pdf (1MB, pdf)