Figure 3.
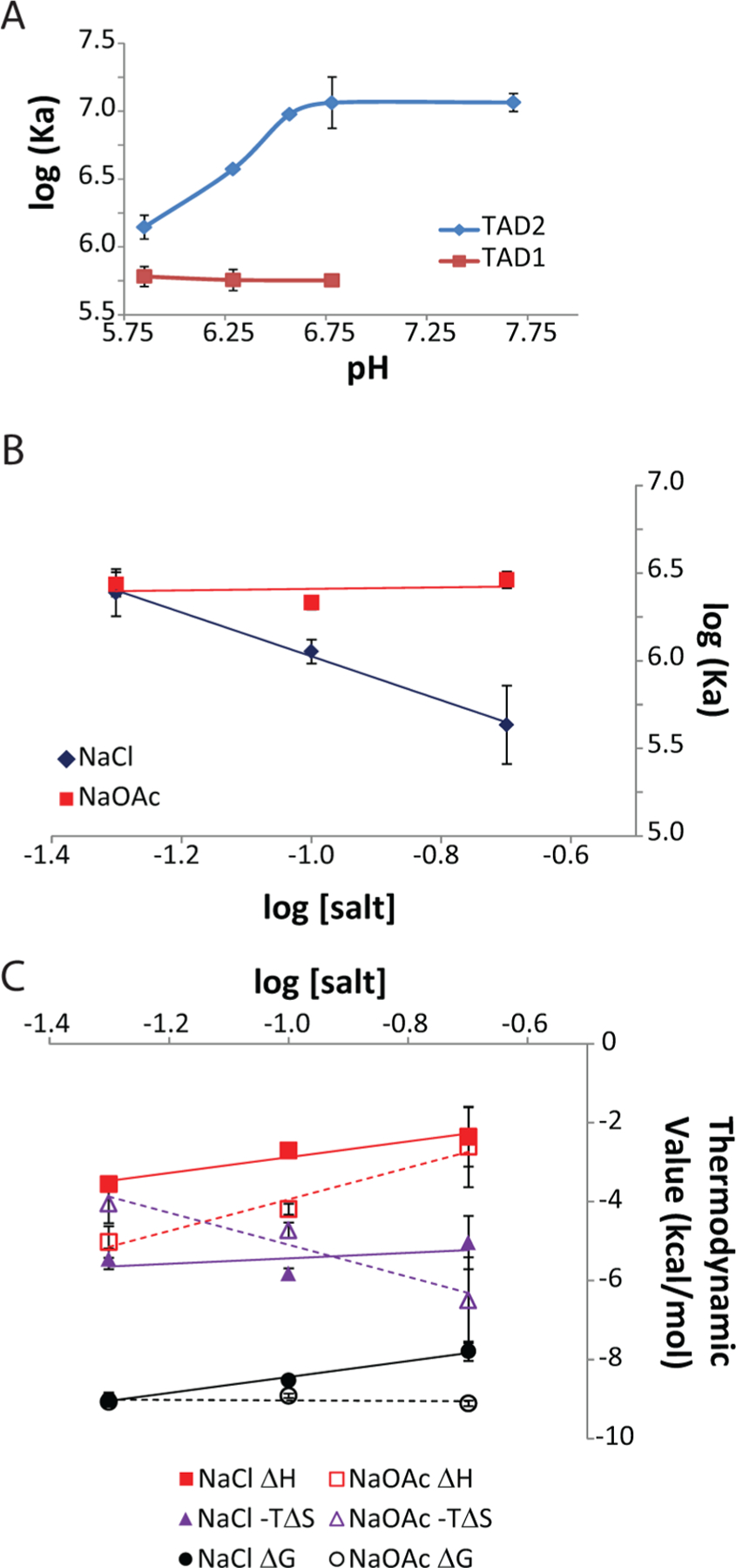
Thermodynamic characterization of the pH and salt dependence of p53–Taz2 interactions. (A) pH dependence of the affinity of the complex formed between Taz2 and p53(1–39) (empty symbols) or p53(35–59) (filled symbols) as measured by ITC at 35°C. Binding experiments were performed as described in Experimental Procedures, with pH values measured at 35 °C. (B) Salt dependence of the binding affinity of the Taz2–p53(35–59) complex as measured by ITC at 35 °C in buffer containing either NaCl (filled symbols) or NaOAc (empty symbols). (C) Salt dependece of the thermodynamic parameters of complexation of p53(25–59) with Taz2 as measured by ITC at 35 °C in buffer containing either NaCl (filled symbols) or NaOAc (empty symbols). Squares, ΔH; triangles, −TΔS; circles, ΔG.
