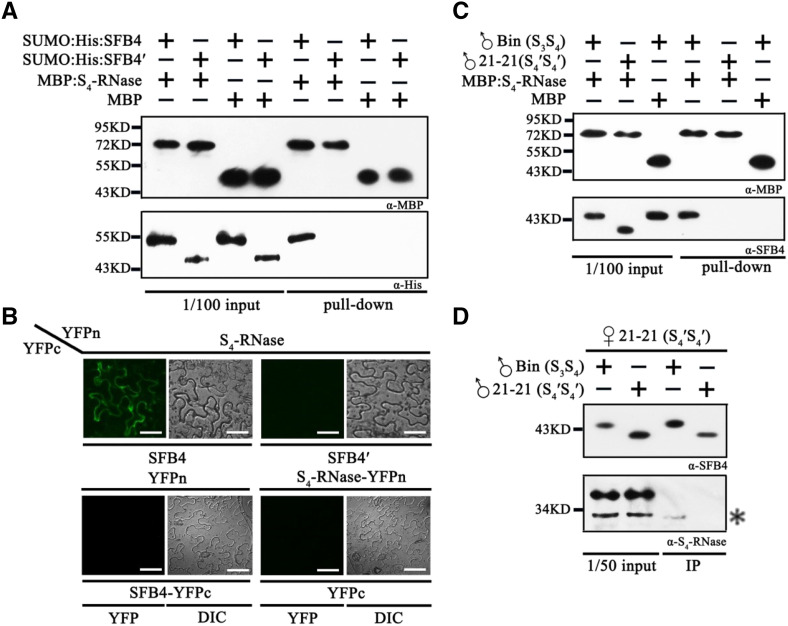
Figure 2.
SFB4′ protein does not interact with S4-RNase. A, Pull-down assay to detect the interaction between S4-RNase and SFB4/SFB4′. SFB4 and SFB4′ were fused with SUMO and His tags, and the molecular masses were almost 55 and 45 kD, respectively. S4-RNase was fused with an MBP tag, and the molecular mass reached around 72 kD. SFB4 and SFB4′ were detected by His tag monoclonal antibody, while S4-RNase and MBP were detected by MBP monoclonal antibody. B, BiFC assay to detect the interaction between S4-RNase and SFB4/SFB4′. S4-RNase was fused with the N-terminal end of YFP, SFB4 and SFB4′ were fused with the C-terminal end of YFP, and the combinations S4-RNase/SFB4, S4-RNase/SFB4′, S4-RNase/YFPc, and YFPn/SFB4 were expressed in N. benthamiana leaves. DIC, Differential interference contrast. Bars = 20 μm. C, Semi-in vivo pull-down assay to further detect the interaction between S4-RNase and SFB4/SFB4′. Pollen tube proteins from cv Bin and cv 21-21 were incubated with MBP protein and MBP-tagged S4-RNase. The molecular masses of SFB4 and SFB4′ were almost 45 and 39 kD in pollen tubes, respectively. D, Semi-in vivo immunoprecipitation (IP) assay to confirm the interaction. Pistil proteins from cv 21-21 were incubated with pollen tube proteins from cv Bin and cv 21-21. The molecular mass of S4-RNase was almost 30 kD. The asterisk represents the signal of S4-RNase. The higher molecular mass band is a nonspecific band. α-His, His tag antibody; α-MBP, MBP tag antibody; α-S4-RNase, S4-RNase antibody; α-SFB4, SFB4 antibody.