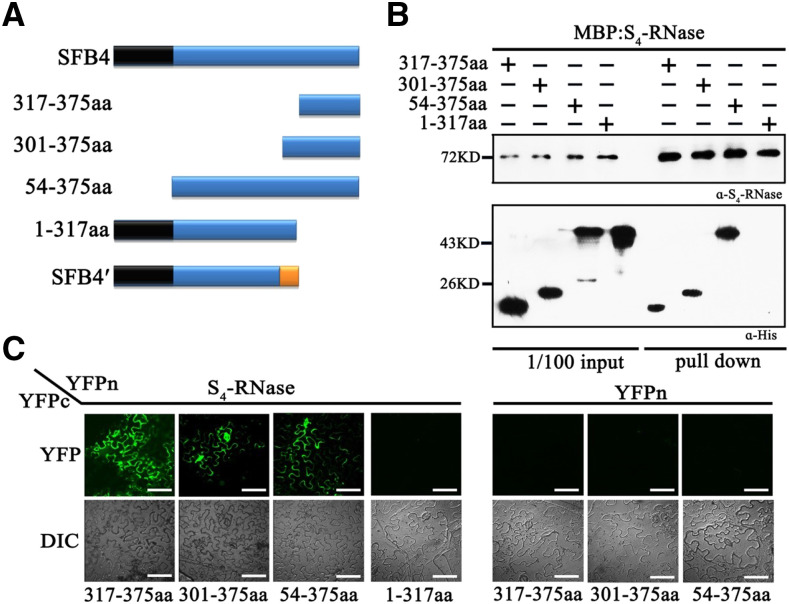
Figure 3.
The SFB4 C-terminal region is important for its ability to interact with S4-RNase. A, Schematic diagram of the four SFB4 fragments (317-375aa, 301-375aa, 54-375aa, and 1-317aa). B, Pull-down assay to detect the interaction between S4-RNase and different SFB4 fragments. S4-RNase was fused to the MBP tag and detected by S4-RNase antibody. The SFB4 fragments were fused to two tags of SUMO and His and detected by His tag antibody. The molecular masses of 317-375aa, 301-375aa, 54-375aa, and 1-317aa fragments were almost 21, 24, 50, and 50 kD, respectively. α-His, His tag antibody; α-S4-RNase, S4-RNase antibody. C, BiFC assay to further confirm the interaction. S4-RNase was fused with the N-terminal end of YFP, the SFB4 fragments were fused with the C-terminal end of YFP, and the combinations S4-RNase/SFB4-317-375aa, S4-RNase/SFB4-301-375aa, S4-RNase/SFB4-54-375aa, S4-RNase/SFB4-1-317aa, YFPn/SFB4-317-375aa YFPn/SFB4-301-375aa, and YFPn/SFB4-54-375aa were coexpressed in N. benthamiana leaves. DIC, Differential interference contrast. Bars = 20 μm.