Figure 3.
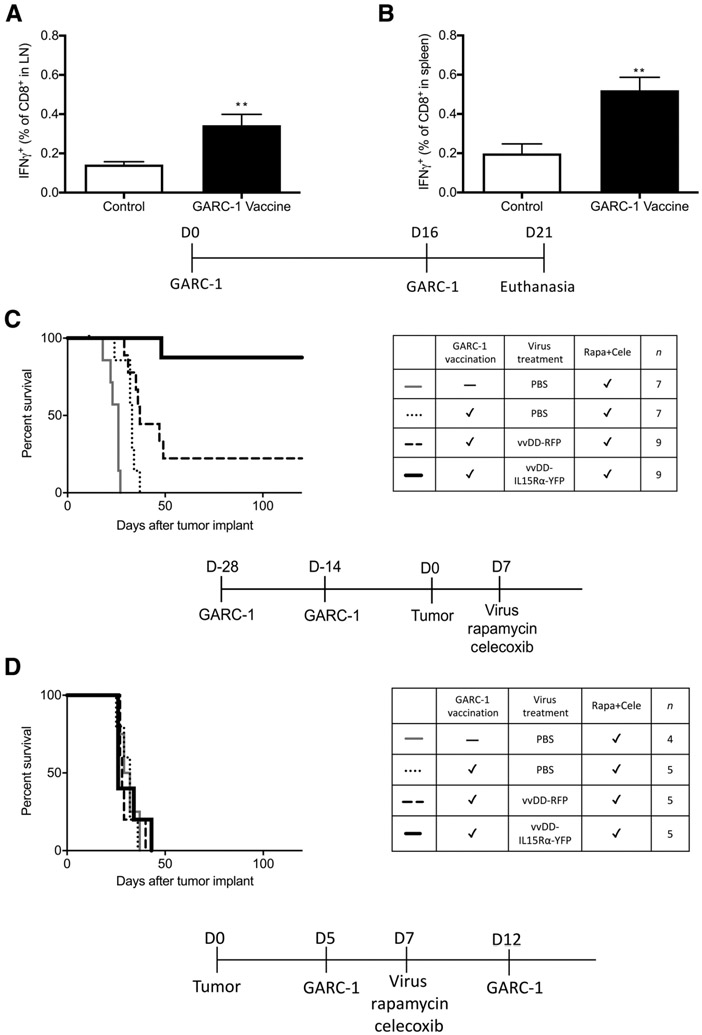
Tumor antigen-specific CD8+ T cells are generated by emulsion vaccination and have the potential to prolong survival of C57BL/6J mice bearing intracranial GL261 NS tumors treated with intratumoral vvDD-IL15Rα-YFP virus, rapamycin, and celecoxib. The vaccine combining 50 μg GARC-1 peptide, 50 μg CpG ODN in 50 μL PBS, mixed with 50 μL IFA for each mouse was emulsified by sonication and injected subcutaneously in left flank (100 μL) followed by a booster injection 16 days later. Five days after the booster injection, cells from axillary and brachial lymph nodes on the left side and spleens were harvested and exposed to GARC-1 peptide followed by flow cytometry analysis of CD8 and IFNγ. A, Lymph node percentage of GARC-1–specific CD8+ T cells among all CD8+ T cells. Mean and SEM from 5 mice per group are shown. **, P < 0.01 for vaccinated group versus control group. B, Spleen percentage of GARC-1–specific CD8+ T cells among all CD8+ T cells. Mean and SEM from 5 mice per group are shown. **, P < 0.01 for vaccinated group versus control group. C, Survival of tumor-bearing mice treated with prevaccination of GARC-1, intratumoral vvDD-IL15Rα-YFP, rapamycin, and celecoxib. Mice received GARC-1 emulsion vaccine on day −28 and −14; tumor on day 0; and 1 μL virus (2 × 106 pfu) or PBS injection, rapamycin, and celecoxib on day 7 (medication treatment continued until day 73). Mice that received the combination treatment lived significantly longer than other groups. P < 0.01 for vvDD-IL15Rα-YFP–treated group compared with each of the other groups. Prevaccinated vvDD-RFP and prevaccinated PBS were also different from unvaccinated PBS-treated mice, P < 0.05. Prevaccinated vvDD-RFP differed from prevaccinated PBS, P < 0.05. D, Survival of tumor-bearing mice treated with postvaccination with emulsion vaccine of GARC-1, intratumoral vvDD-IL15Rα-YFP, rapamycin, and celecoxib. Mice received tumor implantation on day 0; GARC-1 emulsion vaccine on day 5 and 12; and 1 μL virus (2 × 106 pfu) or PBS injection, rapamycin, and celecoxib on day 7 (medication treatment continued until day 73). There was no significant difference between any treatment groups.
