Figure 5.
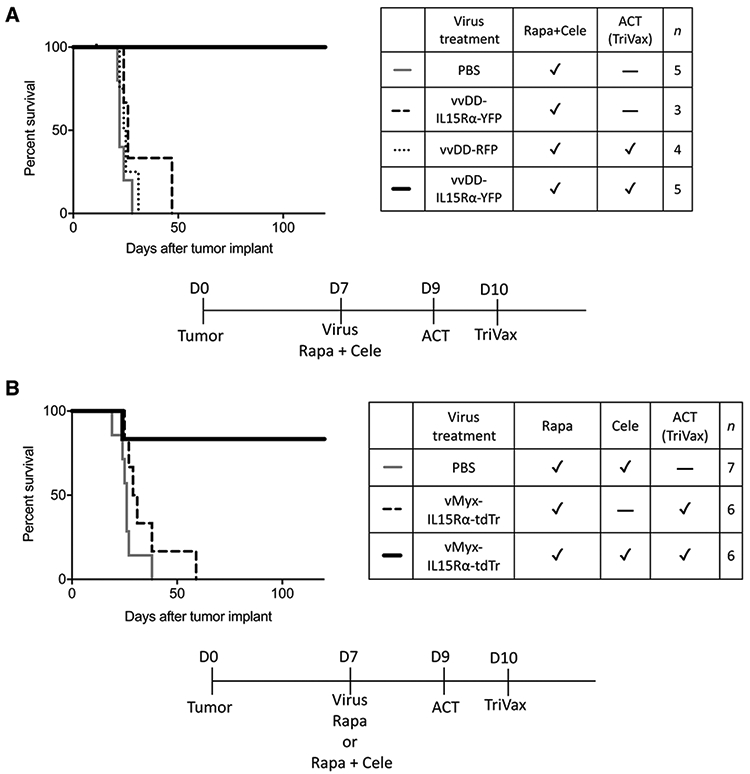
The therapeutic effect of the full combination treatment using vvDD-IL15Rα-YFP or vMyx-IL15Rα-tdTr. A, Survival of tumor-bearing mice treated with intratumoral vvDD-IL15Rα-YFP virus injection, rapamycin and celecoxib, adoptive transfer of CD8+ T cells, and TriVax booster. Mice received tumor on day 0; 1 μL vvDD-IL15Rα-YFP virus (2 × 106 pfu) or PBS injection, rapamycin, and celecoxib on day 7 (medication treatment continued until day 73; adoptive transfer of CD8+ T cells on day 9; and TriVax booster on day 10. Mice that received the full combination treatment survived longer compared with other groups, indicating the requirement for ACT. P < 0.01 for each comparison. B, Survival of tumor-bearing mice treated with intratumoral vMyx-IL15Rα-tdTr virus injection, rapamycin and celecoxib, adoptive transfer of CD8+ T cells, and TriVax booster. Mice received tumor on day 0; 1 μL vMyx-IL15Rα-tdTr virus (2 × 106 pfu) or PBS injection, rapamycin with or without celecoxib on day 7 (medication treatment continued until day 73; adoptive transfer of CD8+ T cells on day 9; and TriVax booster on day 10. Mice that received the full combination treatment survived longer compared with other groups, indicating the requirement for celecoxib and the insufficiency of ACT (TriVax). P < 0.05 for each comparison.
