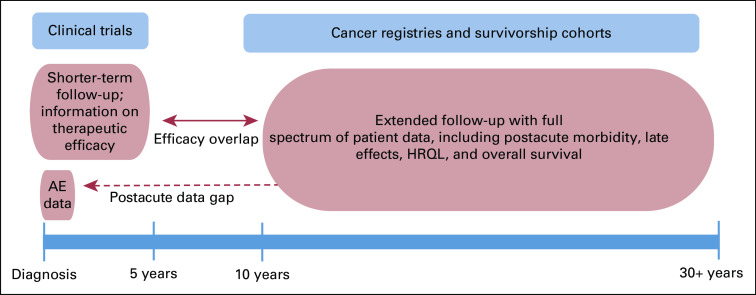
FIG 1.
Data sources to study the continuum of care for Hodgkin lymphoma. Ideal information to study morbidity health-related quality of life (HRQL), and mortality across the lifelong time horizon for patients with classic Hodgkin lymphoma (cHL) is not available from a single source of data. Clinical trials typically examine shorter-term efficacy (3-5 years) and acute on-study adverse events (AEs). Most clinical trials do not track AEs or morbidity that occur post-therapy, which leads to critical data gaps. Cancer registries and survivorship cohorts comprise real-world data that provide important insights with longer-term follow-up for morbidity, HRQL, and excess mortality that manifest after cHL therapy, including postacute outcomes (ie, 1-10 years after diagnosis).