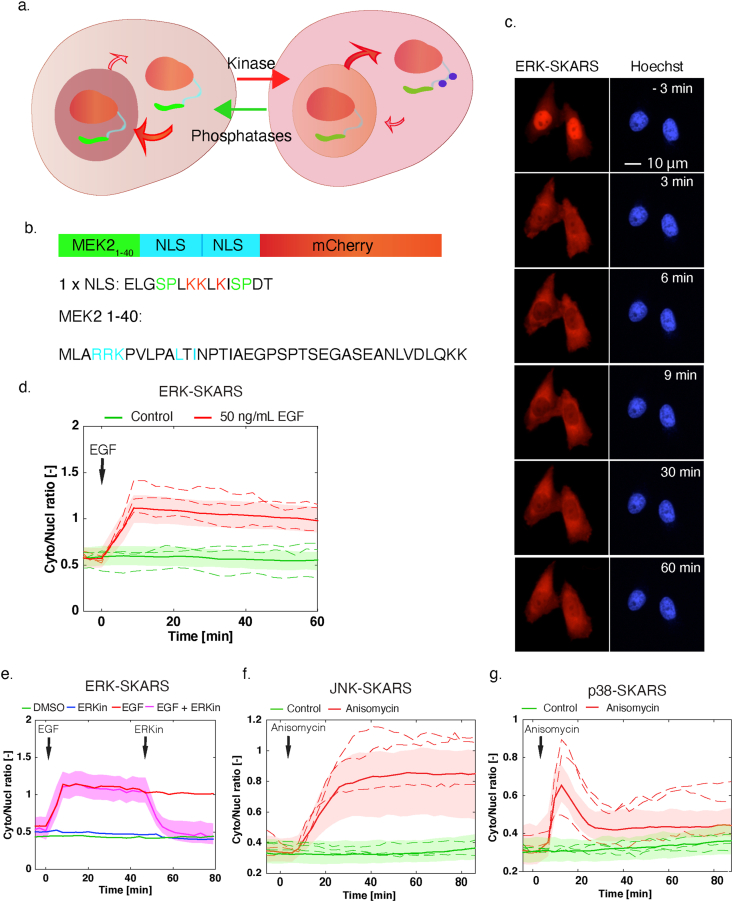
Figure 1.
Principle and development of synthetic kinase activity relocation sensor (SKARS) to monitor MAPK activity in mammalian cells. a. Scheme of the SKARS relocation process. When the kinase is inactive, the NLS is functional and the sensor accumulates in the nucleus. When the kinase is active, it phosphorylates the SKARS, which relocates into the cytoplasm. b. The ERK-SKARS contains three domains: the ERK docking site (MEK2 1–40), the two Nuclear Localization Sequences (NLS), and the fluorescent protein for visualization. Residues involved in interaction, nuclear import or phosphorylation are shown in cyan, red and green, respectively. c. Representative microscopy images of HeLa cells expressing the ERK-SKARS cells and stimulated with EGF (50 ng/ml) for 1 h period. In the red channel, the ERK-SKARS translocates from the nucleus to the cytoplasm. Nuclei are identified by a Hoechst staining. d. After quantification of the time-lapse movies, the ratio of the average cytoplasmic fluorescence of the average nuclear fluorescence (Cyto/Nucl ratio) is plotted as function of time. For all similar figures in this paper, the solid lines represent the median of the cell population and the shaded area the 25 and 75 percentiles of the population. The dotted lines represent a few single-cell traces extracted from the cell population. More than hundred single cells measured were plotted in the graph. The red curve represents HeLa cell treated with 50 ng/ml EGF (Number of cells: Nc = 340) and the green curve, mock-treated control cells (Nc = 449). e. Cyto/Nucl ratios of HeLa cells expressing the ERK-SKARS exposed to EGF stimulation (50 ng/ml) and ERK inhibition (FR 180204, 50 ng/ml) are plotted as function of time. EGF and ERK inhibitor were added at the time points indicated by the arrows. f. and g. HeLa cells expressing the JNK-SKARS (f) and the p38-SKARS (g) were treated with (red) and without (green) Anisomycin (50 ng/ml). The Cyto/Nucl ratio is plotted as function of time.