Figure 1.
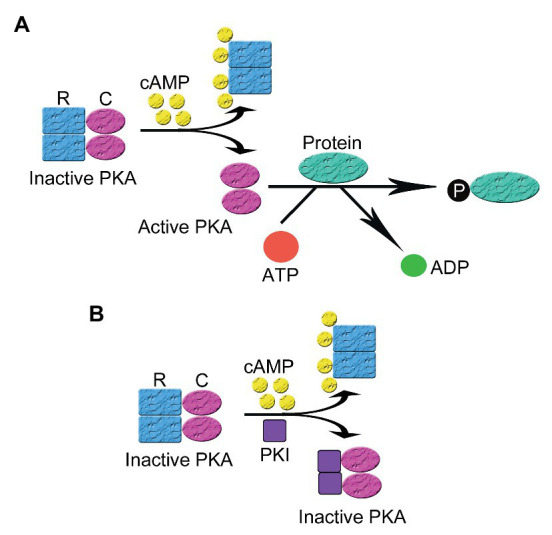
The activation and inhibition of cyclic adenosine monophosphate (cAMP)-dependent protein kinase A (PKA). (A) In the absence of cAMP, PKA is an inactive tetrameric holoenzyme consisted of two regulatory (R) and two catalytic subunits (C). In the presence of cAMP, each R subunit binds to two molecules of cAMP at separate allosteric binding sites, leading to the decrease of the affinity between the R and the C subunits and the separation of the holoenzyme of PKA into a regulatory subunit dimer and two monomeric catalytic subunits. The released C subunits becomes active and phosphorylates their substrates on their serine and/or threonine sites in different signaling microdomains, leading to the conduction of cellular biological function (B) In the presence of cAMP, the regulatory and catalytic subunits that comprise the PKA holoenzyme dissociate. Then protein kinase inhibitor peptide (PKI) inhibits the activity of PKA by binding to the free C subunit of PKA and inhibiting the phosphorylation of PKA substrates.
