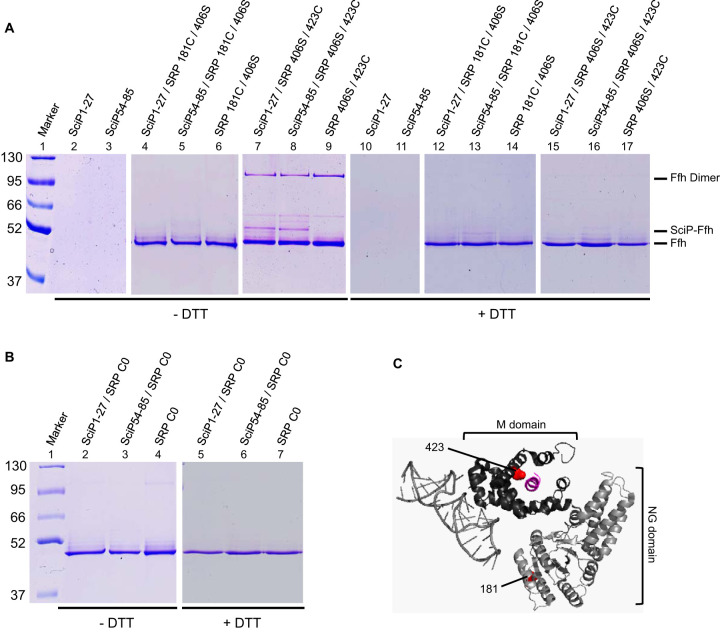
FIG 5.
In vitro disulfide cross-linking of SRP and SciP1-27/SciP54-85 with copper phenanthroline. (A and B) We incubated 20 μM of the SciP1-27 or SciP54-85 peptides (single cysteines at positions 16 and 68, respectively) with 2 μM reconstituted SRP L181C/406S or SRP 406S/423C (A) or SRP C0 (B) and 1 mM copper phenanthroline for 1 h on ice. The samples were TCA precipitated, resuspended in buffer with or without DTT, and loaded on 10% SDS-PAGE. When the peptides were incubated with SRP 406S/423C, a 52-kDa cross-linking band (SciP-Ffh) appeared. The shifted bands were sensitive to the reducing agent DTT. No cross-linking band appeared with SRP 181C/406S and SRP C0. The SciP peptides and the SRP mutants alone were analyzed for control. (C) Crystal structure of the E. coli SRP bound to a signal sequence, displayed as a cartoon. The 4.5S RNA and the Ffh NG domain are colored in light gray, the Ffh M domain is in dark gray, and the signal sequence is in purple. The positions of the incorporated cysteine residues are displayed as red dots (image created with PyMOL 2.3.0; PDB ID 5GAD).