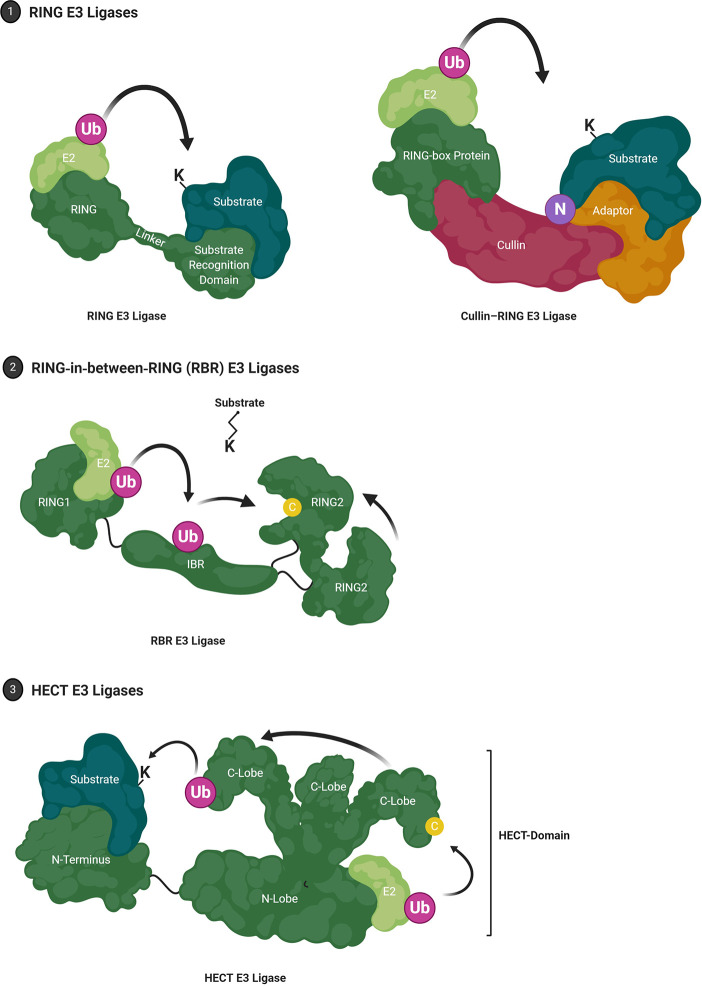
Figure 2.
E3 Ubiquitin Ligases. (1) RING (Really Interesting New Gene) E3 ligases facilitate Ub-E2:substrate interaction. RING E3 ligases may also organize into multi-subunit complexes that are commonly composed of Cullins, E2 binding RING-box proteins and an adaptor protein that mediates substrate recognition. Canonically, neddylation (NEDD8) is required to induce the active conformer. (2) RBR (RING-in-between-RING) E3 ligases constitute a hybrid class between RING and HECT E3 ligases where the RING1 domain facilitates E2 interaction while the RING2 domain harbors a catalytic cysteine residue that forms an intermediate thioester. (3) HECT (Homologous to E6-AP Carboxyl Terminus) E3 ligases are characterized by a conserved bi-lobed, catalytic HECT domain. The loaded E2 is bound by the N-lobe where ubiquitin is transferred to the catalytic cysteine residue on the flexible C-lobe. The C-lobe-ubiquitin thioester intermediate rotates toward the substrate which is bound by a substrate-binding domain located N-terminal of the HECT domain.