Abstract
Cancer-testis antigens (CTA) are tumor antigens, present in the germ cells of testes, ovaries and trophoblasts, which undergo deregulated expression in the tumor and malignant cells. CTA genes are either X-linked or autosomal, favourably expressed in spermatogonia and spermatocytes, respectively. CTAs trigger unprompted humoral immunity and immune responses in malignancies, altering tumor cell physiology and neoplastic behaviors. CTAs demonstrate varied expression profile, with increased abundance in malignant melanoma and prostate, lung, breast and epithelial cell cancers, and a relatively reduced prevalence in intestinal cancer, renal cell adenocarcinoma and malignancies of immune cells. A combination of epigenetic and non-epigenetic agents regulates CTA mRNA expression, with the key participation of CpG islands and CpG-rich promoters, histone methyltransferases, cytokines, tyrosine kinases and transcriptional activators and repressors. CTA triggers gametogenesis, in association with mutated tumorigenic genes and tumor repressors. The CTAs function as potential biomarkers, particularly for prostate, cervical, breast, colorectal, gastric, urinary bladder, liver and lung carcinomas, characterized by alternate splicing and phenotypic heterogeneity in the cells. Additionally, CTAs are prospective targets for vaccine therapy, with the MAGE-A3 and NYESO-1 undergoing clinical trials for tumor regression in malignant melanoma. They have been deemed important for adaptive immunotherapy, marked by limited expression in normal somatic tissues and recurrent up-regulation in epithelial carcinoma. Overall, the current review delineates an up-dated understanding of the intricate processes of CTA expression and regulation in cancer. It further portrays the role of CTAs as biomarkers and probable candidates for tumor immunotherapy, with a future prospect in cancer treatment.
Keywords: CTA, expression, functions, cancer diagnosis, immunotherapy, oncogenic targets
Introduction
Resistance to chemotherapy, radiation therapy and monoclonal antibody-based therapy is common in cancers, malignancies and metastatic conditions in children, as well as adults, with very few therapeutic alternatives. On the other hand, immune-based treatments have led to the concept of T lymphocyte-mediated cancer antigen specificity, where Cancer testis antigen (CTA)-based therapy appeared important for treating cancers. Most importantly, the T lymphocytes were capable of recognizing CTAs, and the latter induced strong antitumor immune responses [1,2].
CTAs comprise an assembly of tumor-associated proteins, which undergo tumor-restricted expression and further elicit unprompted immune responses in cancer patients [3,4]. Tumor mutation burden (TMB) bears a strong relationship with response and prognosis of cancer immunotherapy, and CTAs demonstrate a significant increase in patients with elevated TMB [5,6]. CTAs are localized on the X chromosome, over-expressed in male germ cells and generally expressed by gametes and trophoblasts [7,8]. CTA family members are prevalent in cancerous cells from varied histological types, and exhibit non-specific expression and distribution in different cell lineages. Several of them show partial or significant sequence homology as well [7]. They are aberrantly activated in diverse human malignant cells and rarely in the reproductive cells. Additionally, they show strong link with hormone imbalances associated with aggressive forms of cancers, mainly in patients with reduced chances of survival [5].
The CTAs are predominantly suppressed through DNA methylation in vegetal cells and undergo epigenetic activation in malignant tumors [9]. The epigenetic processes up-regulate after chemotherapy, promoting CTA-specific vaccine-mediated reduction in tumor growth and survival [10]. Moreover, these tumor-associated antigens, although do not directly participate in disease development and progression, play a key role in signal transmission gene orchestration and oncogenic modulation [11,12]. The CTAs provide signals for cell division, proliferation, growth and aggressive tumor behaviour [12-14]. By altering the transcriptional and post-transcriptional machinery, CTAs regulate gene expression for germ cell multiplication [15,16]. CTAs present specific features of neoplasia, including immune response evasion, perpetuity, DNA hypomethylation, epigenetic abnormality, invasiveness and destruction of healthy tissues [17,18]. Moreover, the cancer-immune interactions increase metastatic spread and communication to new hosts [19].
The CTA concept first appeared in 1960s with the serological recognition of liver cancer and the ovarian or testicular germ cell tumor marker, alpha-fetoprotein [20]. In line with this, mice with human carcinoembryonic antigen (CEA) transgene served as preclinical models for immunotherapy, particularly at the luminal epithelial cells of cecum and colon [21]. However, only during 1990s, the idea of adaptive immune responses received recognition, and Boon and colleagues in 1991 identified the first CTA, Melanoma-Associated Antigen 1 (MAGEA-1), via cloning of genes encoding T-cell epitopes [24].
In this review article, we summarize the current role of CTA in oncogenesis, and discuss the participation of CTA-based approach, individually and in combination with other immunotherapies, as a novel therapeutic strategy for cancer. We enlighten the urgent need to explore novel opportunities for early detection and prognosis for cancers, where CTAs may play a critical role. We also illustrate the advances made over the years towards development and improvement of CTA-based therapies, and the need for newer and more specialized CTAs, specific for certain cancers and malignant subtypes.
CTA: types and characteristics
CTA genes are generally located on the X-chromosome, and form 10% of its coding sequence [25]. They bear germ-cell specificity and have the ability to provoke humoral and/or cell-mediated immune responses [26]. Several CTAs have been discovered by T-cell epitope cloning, which mainly belong to the Chromosome X-encoded (CT-X) category (Table 1). CT-X antigens are primarily expressed during embryonic growth and spermatogenesis [27]. They help in spermatozoa development from mitotic and proliferating germ cells in columnar Sertoli cells of the testis. Several of them bunch together at the telomeric long arm (q) ends, between q24-28, and protect the male germline against stress [27-29]. Genomic analysis reveals that ‘inverted’ or ‘direct’ repeat sequences flank the Xp11 and Xq26-q28 domains of CTX genes. These repeat sequences are widely distributed in the non-XT CTAs as a single copy in abnormal conditions. Conversely, CT-X genes are precisely expressed in the seminiferous tubules of testis at the earlier stages of spermatogenesis [26].
Table 1.
Important CT-X types of CTA, expression site and functions
| CTA-Type | Expression site in normal tissue | Expression site in malignant tissues | Function |
|---|---|---|---|
| MAGEA | Less in normal tissues | Human neoplasms of breast, skin, glioma, neuroblastoma, non-small cell lung cancer, intestine, colorectal, ovary and the kidney | For early diagnosis of site specific malignancy and immunotherapeutic target |
| NY-SO-1 | Synovial membrane and placenta | Breast, neuroblastoma, leukemia, advanced melanoma, urinary bladder, Bone, esophagus, liver and head and neck cancers, non-small cell lung cancer and ovarian cancers | Functions in cell cycle evolution and development |
| SSX | Testis germline tissue, mesenchymal stem cells and melanoma stem cells | Testis, at the spermatogonia stage, synovial sarcoma, head and neck cancer, urinary bladder, ovary, malignant melanoma, prostate gland, colon, intestine, breast, lung, glial, neuronal, endometrial and kidney cancers | Important target for tumor immunotherapy |
| SAGE1 | Central nervous system, hip and thigh bone marrow | Head and neck squamous cell carcinoma, bone and connective tissue, ovaries and testes, myeloma and digestive tract cancers | Detect of malignant squamous lesions, and functions as biomarker |
| CT45 | Spermatogonia and spermatocytes | Hodgkin’s lymphoma, ovarian cancer, germ cell tumors and multiple myeloma | Stage specific biomarkers for site specific malignancies |
| PAGE5 | Hepatic cell, epithelial layers of skin and placenta | Testis and liver cancers and advanced melanoma | Promotes survival of melanoma cells and hence, a potential drug target |
| NXF2 | Bronchial region, pulmonary tissue, placenta and epithelial layers of skin | Lung, urinary bladder, bone and soft connective tissue cancers | Linked with the cell nucleus, and hence functions as effective nuclear targets in cancer therapy |
| SP17 | Male germinal cells | Ovary, breast, esophagus, glial and neuronal cancers and multiple myeloma | SP17-specific cytotoxic T lymphocytes inhibit breast cancer cell proliferation and promote cell death |
The CTAs comprise a total of more than 200 proteins, namely, GAGE, synovial sarcoma-X chromosome (SSX), MAGE, SAGE, CTA-45 (CT45), etc. They are found in pre-meiotic germ cells in adult testis, post-meiotic germ cells, placenta and trophoblastic tissues, where they play a critical role in modulating cancer initiation and progression [12]. MAGE-1 was the first identified CTA from melanoma patients. Following this, the other immunogenic CTAs came into prominence, detected via molecular characterization of T-cell clones that react with autologous human tumors, as well as serologic tests of cDNA and DNA-cloning methodology [30-32]. MAGE-like genes have been detected in non-mammalian species, such as fruit fly and Zebra fish, and not in Roundworm and yeast, suggesting the evolutionary conservation of the gene [17,33]. Via interaction with pro-caspases and the ‘Really Interesting New Gene’ domain (zinc finger type), MAGE-I proteins modulate E3 ubiquitin ligase activity. The MAGE-RING ligase assembly further participates in several cellular processes, including membrane trafficking, DNA damage repair and signaling mechanisms [34]. Some supplementary CTAs, such as, BAGE, HAGE, Sperm Protein 17 (SP17) etc. are common across angiosperm genomes. They are generally localized on the autosome pairs, bear homologous alleles and are of same size [35]. The additional subclass of CTAs, composed primarily of proteins encoded by single-copy genes located on the autosomes, are mainly expressed in the meiotic and post-meiotic male germ cells and participate in gene transcription [35,36]. They play a key role in spermatogenesis and male fertility [37]. The New York esophageal squamous cell cancer-1 (NY-ESO-1) type of CTA retains robust spontaneous immunogenicity, elicits cellular and humoral responses and exhibits restricted expression pattern [38]. Moreover, the atypical expression of Non-XTs in malignant cancer cells results in chromosomal abnormalities [39]. The Non-XT types of CTA, such as, SPO11, A disintegrin and metalloproteinase (ADAM) domain 2 (ADAM2), centrosomal protein of 55 KDa (CEP55), Kinetochore protein Nuf2 and TTK protein kinase (Table 2) are normally dispersed along the whole genome, and fail to localise in long repeats of genomic sequences and establish gene families. They are mainly located in the testes at the advanced stages of spermatogenesis and participate in male germ cell differentiation at seminiferous tubules. CEP55 takes part in spermatogenesis, and ADAM2, in association with the other ADAMs, contributes towards Nuf2-regulated oocyte meiosis and sperm-egg adhesion and fertilization [40-42]. The X-CT and non-XT groups of CTA, generally expressed at discrete stages of spermatogenesis, engage in the diverse cellular events related to spermatogenesis [7,43].
Table 2.
Important Non-XCT types of CTA, expression site and functions
| CTA type | Expression site in normal tissues | Expression site in malignant tissues | Function |
|---|---|---|---|
| SPO11 | Central nervous system, epithelial cells and muscle tissue | Melanoma, lung, cervical and breast cancers | Regulates genomic stability during tumorigenesis, and these proteins, therefore, represent promising targets for novel therapeutic strategies |
| ADAM2 | Prostate gland, central nervous system, epithelial cells and muscle tissue | Head and neck squamous cell carcinoma, colorectal cancer, leukemia, prostate, glioma and connective tissue cancers | Functions as a cell matrix protease in the growth, development and propagation of cancers |
| CEP55 | Spermatocytes, prostate gland and testis | Breast, colorectal, liver, lung, endometrial and prostate cancers | Mitosis and cytokinesis in carcinogenesis |
| NUF2 | Prostate gland cells | Metastatic prostate and lung cancers | Target for immunotherapy |
| TTK | Normal colon epithelium | Esophageal squamous cell carcinoma (ESCC), multiple myeloma, lung, breast and colorectal cancer | Cytotoxic activity against ESCC cells |
Additionally, based on expression and localization, CTAs have been categorized as testis-specific (such as, MAGE-A1 and MAGEA2), testis-selective (in testis and a maximum of two more tissues, BAGE and NY-ESO-1) and testis and central nervous system (MAGE-A9)-specific [44]. Malignancies have also been categorized as CT-rich (urinary bladder, skin, ovarian cancers and Non-small-cell lung carcinoma), CT-intermediate (breast and prostate cancers) and CT-poor (hypernephroma, bowel cancer and leukemia), depending on CT-enrichment [45]. Currently, tumorigenesis and oncogenicity are considered as key characteristics of CTA. CTAs are tumor-specific and immunogenic, and generally increase the proliferative, anti-apoptotic, invasive, metastatic and pro-angiogenic features of cancer cells. Thus, it has been hypothesized that CTA-based vaccination, adoptive T-cell therapy or CTA in combination with chemotherapeutics may serve as potential approaches for cancer treatment.
Modulation of CTA expression
The appearance of CTA in cancers depends on its gene status, or more specifically, transcript condition. In chronic myelogenous leukemia (CML), specific CTA genes undergo up-regulation from early stages to the blast phase of the disease [26,46]. The CTAs exhibit tumor-specific expression, showing less than 20% of MAGE-A1 levels in primary malignant melanoma and >50% in metastatic skin cancer [47]. The expression of MAGE-A1 has a significant link with growth and metastasis of malignant melanoma, patient survival rate and gene expression of the leucine-rich members of Preferentially Expressed antigen In Melanoma (PRAME) family [48,49]. Notably, CTA expression levels do not necessarily correlate with the oncogenicity, exemplified by the disparate expression patterns of MAGE-A1 in early and late stage skin cancer, while NY-ESO-1 level remains unaltered [31].
The CTAs are mainly meiotic germ cell antigens, expressed primarily in seminiferous tubules of testis at the formative stages of spermatozoa and in male gametocytes [50]. However, an inadequate expression of CTA has been reported in the cytoplasm of male germ cells [7]. Compared to mRNA, studies on CTA proteins are very rare, and owing to well-controlled post-transcriptional and translational machinery, a >10% expression of particular CTA mRNA guarantees significant protein levels. CTA expression shows uniformity in clonal and subclonal expansions, while an assorted CTA expression involves epigenetic changes in the cancerous cells [17,28]. Hence, a combination of epigenetics and immunotherapy serves as a potent cancer management strategy [51].
CTA genes bear intertumor diversity, owing to the global pattern of DNA methylation or gene-specific promoter methylation, specific gene activation and repression at discrete steps in gametogenesis and trophoblast maturation [52]. A complex interface among the DNA sites, together with distinct nucleic acid-associated histones and transcriptional activators drive the CTA gene transcription. Moreover, cell or tissue-specific configurational alteration of DNA along with chromatin condensation and CpG island methylation by DNA methyltransferase enzymes and S-adenosyl-methionine regulate transcription and translation of CTA. Histone methylation and acetylation direct trascriptional activation of CTA genes, via histone methyltransferases, histone deacetylase enzymes (HDACs) and methyl-CpG binding proteins. Additionally, histone deacetylation restricts the RNA polymerase reaction and transcriptional events, attenuating CTA expression. The methylated promoters also control the expression of C-T genes [9,31,53].
Epigenetic mechanisms regulate the functions of cytotoxic T lymphocyte (CD8+) or helper (CD4+) T-cells, increasing probability of CTA-targeted immunotherapy in patients diagnosed with melanoma [54]. In fact, promoter demethylation increases the expression of NY-ESO-1 and MAGE-A3, which correlate with mitotic and meiotic deformities and irregularities in myeloma. Overall, global hypomethylation and repeated DNA elements in the malignant cells and tissues highlight the critical participation of DNA methylation in modulation of CTA expression. This draws support from transfection-based reporter gene expression studies, demonstrating the precise role of epigenetic regulatory mechanisms for CTA expression in normal and abnormal tissues [26].
Tumors predominantly express CTAs, with the epigenetic pathways being key interconnecting regulators. A few non-epigenetic conditions, particularly the signaling pathways, modulate CTA levels as well. The notable ones include the cytokines, interleukin (IL)-7 and Granulocyte-Macrophage Colony Stimulating Factor, that enhance the CTA, SPAN-xb and SEMG1 levels [55]. CTA genes themselves also control the transcription of other CTA genes. It has been observed that Brother of the Regulator of Imprinted Sites (BORIS) plays an important role in testes-specific protease 50 (TSP50) expression. CTA activation also causes a shift in between the activator and repressor proteins in gametogenesis pathway. Additionally, the Cyclic-Adenosine monophosphate, Krüppel-like factor and Specificity protein family of transcription factors direct MAGE11 and NY-ESO-1 expression. CTA even functions as the master gene during cell development by controlling gametic meiosis and mitosis [56,57].
However, even though CTAs have been considered as potential targets for cancer immunotherapy, methylation and demethylation often suppress the expression of CTA genes. Hence, a regulated methylation of CTA gene promoters and histone post-translational modification appears essential for cancer immunotherapy.
CTAs: role and functions
Synaptonemal complex protein 1 (SCP-1) is a member of the CTA family, which participates in chromosome pairing, synapsis and recombination. It bears critical and specific functions during meiosis in the neoplasms and malignancies of the brain, breast, urinary bladder and epithelial ovarian cancer. SCP-1 expression is regulated by MiR-124 expression, particularly in the neuroglial cells [58].
P-element induced wimpy testis (PIWI), first identified in Drosophila melanogaster, is a class of gene encoding regulatory proteins that maintain the differentiation and self-renewal status in stem cells [7]. PIWI is classified as CTA and is also considered a pro-malignant gene. It mediates its functions via anti-apoptotic and pro-proliferative mechanisms (Figure 1), involving the Signal Transducer and Activator of Transcription-3-regulated mitochondrial B-cell lymphoma-extra large pathway. PIWI, and specifically piwi-like RNA-mediated gene silencing 2 (Piwil2), induces changes in chromatin compaction and alters DNA repair mechanisms. An up-regulated Piwil2 also reduces cellular sensitivity to anti-neoplastic drugs, and mainly chemotherapeutic cisplatin [59,60]. Hence, suppression of Piwil2 reduces oncogenesis and the unrestricted proliferation of cancerous cells, and has been proposed as a prospective immunotherapeutic target and biomarker. PIWI belongs to the Argonaute protein family, which is highly conserved among species and functions as the catalytic component of the RNA-induced silencing complex that sustains cell homeostasis [61]. Argonaute proteins interact with the small non-coding RNAs such as microRNAs (miRNAs), small interfering RNAs (siRNAs) and Piwi-interacting RNAs (piRNAs). PIWI is abundant in the male reproductive cells, mainly confined to the undifferentiated male germ cells (spermatogonia) and early male gametocytes. PIWI is also well-expressed in germ cell tumors of the testicle and infrequently in the mediastinum and extra-gonadal locations [62]. PIWI participates in malignant growth of prostate, breast, intestine, ovary and uterine cancers. Even the murine homolog of Piwi (Miwi) shows distinct expression in the cytoplasmic domain of primary spermatozoa, playing an important role in evolution of haploid spermatozoa from testicular somatic cells [63,64]. However, advancement in the field of next-generation sequencing technology may be utilized for understanding the divergent expression of piRNAs/piwi proteins, which could distinguish between malignant and benign stages [60].
Figure 1.
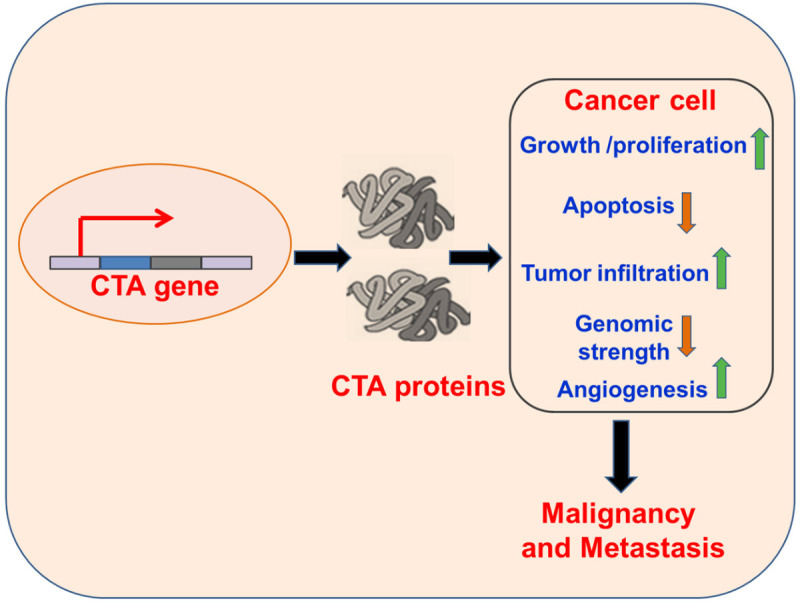
Mechanisms of CTA-based Oncogenesis. The CTAs induce growth and proliferation of tumorigenic cells, reduce cell death via decreased apoptosis, disrupt the genomic strength and promote cellular migration, promoting cellular malignancy and metastasis of malignant cells.
CTAs: gametogenesis and malignancy
Growth and development of germ cells and tumor follow a common pattern, giving rise to John Beard’s ‘trophoblastic theory of cancer’. The theory predicts that CTAs originate from germ cells, mainly found in melanomas and varieties of other tissues, and are absent in normal tissues other than testis [64]. Although, the key relation between gametogenesis and cancer remains obscure, “genome instability in cancer” is termed as one of the critical links. A shared process has been observed among the ploidy sets in cell division, with elevated genomic content in well-developed tumorous and cancer cells that typically mark the CTAs [65]. CTAs are exclusively evident in the undifferentiated spermatogonia and seminomas, while having no expression in the Leydig and Sertoli tubular cells and non-seminomas [33]. The CTA expression levels vary with the spermatogenic phases. CTA also participates a wide range of functions, ranging from mitotic cell cycle progression, spermatogonia, meiotic prophase during the first cycle of spermatogenesis and completing sperm capacitation and acrosome reaction (Figure 2) [7,66].
Figure 2.
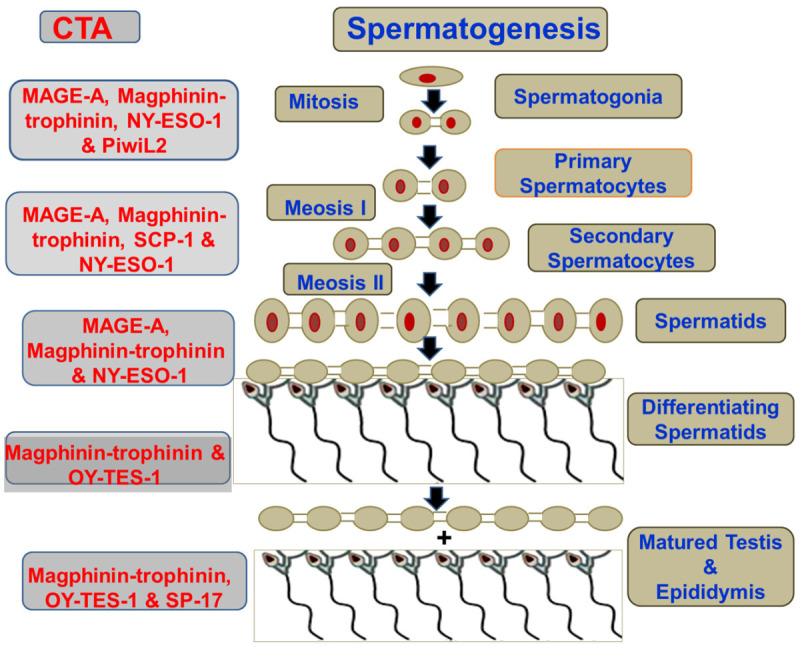
Stage-wise CTA expression during Spermatogenes. The CTAs (LHS) that contribute to the step-wise genesis from germ cells to Spermatozoa (RHS).
The abnormal expression of germline genes during cancer results from the formation of mature haploid gamets, associated with the up-regulation of the otherwise silent germline appearances that drives the process of tumor generation [67]. CTA genes have key functions in malignant as well as testicular germ cells, with distinct regulatory activities governed by overlapping molecular mechanisms [16]. The common traits in cancer as well as germ cells and in gamete and trophoblast differentiation processes include cell replication, transformation, migration, aneuploidy, meiosis and an ultimate metastasis. Additionally, the key features of cancer cells and primordial germ cells comprise stage-specific demethylation, blood vessel formation, suppressed immune responses and expression of human placental hormones after implantation [68].
With the help of demethylating agent, 5-aza-2-deoxycytidine (5DC) that sequesters DNA, CTA could be induced in the cancerous cells, indicating the necessity of down-regulated methylation and transcriptional processes for reduced CTA expression [69]. HDAC inhibitors along with 5DC further up-regulated expression levels of CTAs, with the induction of MAGE, SSX and NY-ESO-1 family members [22]. Epigenetic modifications helped in the expression of CTAs and had key functions as adjuvants for tumor vaccine therapies. Hence, an increased gametic recapitulation could arbitrate cancerous phenotype, even without the oncogenes and anti-oncogenes [70].
A significant variation exists in the expression of X-types of CTA, showing higher expression in the urinary bladder, lung, ovary, liver and melanocyte cancers, and lesser abundance in kidney, intestine, stomach and lymphocyte cancers. The normal somatic cells undergo activation via CpG island hypomethylation, and the CTX genes regulate gene expression and modulate the sensitivity of malignant cell lines to cell growth, cell multiplication, toxic influences and cell death [71]. Here, methylation has been considered as a possible mechanism for controlling tissue-specific expression of CTA genes. Starting from the primordial state, germ cells grow and extend along dorsal mesogastrium into the fetal gonads during development of internal genital tract and external genitalia [7]. Moreover, primordial germ cells turn into prospermatogonia via testicular somatic cell regulation and secretion and transfer of nutrients and regulatory factors [72,73].
SCP-1 is a type of CTA that participates in the gametogenesis process for synapsis during cell division. OY-TES-1 functions in acrosin zymogen packaging and concentration in the acrosome of sperm heads. SPO11 initiates DNA double strand breaks in meiosis and BORIS regulates MAGES and drives tyrosine kinase activity in multilayered germinal epithelium containing spermatogenic and sertoli cells. CTAs act as strong targets for immunotherapies and have been considered for clinical trials in vaccine therapies, particularly because of their immunogenic properties and tumor-dependent expressions [7]. Members of ADAM family also function in sperm-egg contacts, intracellular signaling, cytoskeletal organization of cell-cell and cell-matrix interactions, including sperm-egg fusion, muscle hypertrophy and neural stem cell to neuron generation [74]. The CTA, SP17, found in multiple myeloma, ovarian cancer, brain cancer, throat cancer, endometrial cancer, cervical cancer, glioma and squamous cell carcinoma, does not express in the non-metastatic parental line. Being evident in the human spermatozoa, SP17 undergoes expression in the fibrous sheath of the tail. A-kinase anchoring protein 3 (AKAP3) in the sperm flagella functions along with SP17, which when expressed in tumors results in grave prognosis and remote chances of survival, particularly in ovarian cancer [10]. AKAP usually forms a complex with the SP17 auto-antigen, helping in site-specific sequestration of signaling molecules in both the germ cells and malignant cells. SP17 together with AKAP participates in the fertilization of sperm and ovum during acrosomal reaction at the zona pellucida surrounding the developing oocyte. It also enhances the process of cell-cell matrix interaction, adhesion and migration [10]. SP17 had been initially considered as a prime therapeutic target for gynaecological cancers; nonetheless, its prominent expression in the reproductive as well as non-reproductive cells, such as respiratory epithelial cells, inflammatory cells inside synovial joints and dermal melanophages in both male and females predicted its use as an immunotarget for gyencological cancers only [33].
CTAs are inherently disordered proteins, and participate in a range of functions, including cell signaling, cell differentiation, cell cycle regulation and several other functional pathways. CTA-mediated actions are well-synchronized, being accurately and compactly controlled and directed by a wide-range of post-translational processes, such as, phosphorylation, acetylation and glycosylation, and most importantly alternative splicing [75]. CTAs are capable of intramolecular interaction, resulting in assimilating and construing physiological inputs in a dose-dependent pattern, which are also well-controlled by the conformational dynamics and ensemble of CTAs. These post-translational modifications are critical in helping CTAs to distinguish and separate the different stages of cancers [76].
NY-ESO-1, first detected in a New York City-based lady suffering from squamous cell carcinoma of the oesophagus, is abundantly expressed in the undifferentiated male germ cells and in diploid primary spermatocytes within the testis, and absent in post-meiotic haploid male gametids [77]. Phase-1 clinical trials on histidine-labeled recombinant NY-ESO-1 showed its increased levels in the serum of lung cancer, thyroid cancer, ovarian cancer, breast cancer, urinary bladder cancer, esophageal cancer and melanoma patients [77]. Moreover, a combination of the cancer-targeted monoclonal antibody, ipilimumab, with NY-ESO-1 vaccine had a synergistic therapeutic effect in advanced metastatic skin cancer [38]. It has also been seen that vaccinating NY-ESO-1, together with the target specific adjuvants and potent Type 1 T helper-like immune enhancers, promoted longevity in patients with skin, lung, ovarian, breast and even bone and soft connective tissue cancers [78]. Nonetheless, another clinical study using hydrogel nanoparticles of hydrophobized cholesterol-bearing pullulan and NY-ESO-1 protein vaccine demonstrated increased thymus specific CD4 and CD8 T cell subsets and associated immune responses. It also showed enhanced expression of several other tumor antigens in the peripheral blood, and had no therapeutic effect on tumor volume. Additionally, the patients exhibited increased death rate, proving the combination treatment as futile [79].
CTAs help in cellular migration, proliferation, tumor invasion and penetration, and an ultimate secondary metastatic growth. Notably, NY-ESO-1 undergoes significant up-regulation in advanced skin cancer. Moreover, SSX and N-RAGE-CTAs demonstrate an enhanced expression in multipotent stromal mesenchymal stem cells and suppression in differentiated daughter cells [80]. SSX expression governs the shift between epithelial cell layer and mesenchymal stromal cell formation. Here, reduced SSX together with decreased vimentin structural protein and matrix metalloproteinase 2 promote cellular invasion and attenuate carcinogenesis. The proto-oncogene, CTA45, (expressed in the breast cancer cells) and related CTAs, such as MAGE-D4B, piwil2, SSX, CAGE and CT45A1 that activate mitogen-activated protein kinase and cAMP Response Element-Binding Protein induce epithelial-mesenchymal transition and augment carcinogenesis and metastasis [81].
Thus, methylation/demethylation, vasculogenesis and immune evasion are some common characteristics of gametogenesis and tumor formation. The CTAs, expressed in the spermatogonia, spermatocytes and spermatids, play a contributory role in malignancy and metastasis. Hence, genetic alterations in cancer and CTA gene expression activate the germline expression process, triggering tumor progression and oncogenesis.
CTAs as potential biomarkers for detection and diagnosis of cancers
A custom DNA microarray showed an increase in MAGE-A/CSAG in castrate-resistant prostate cancer (PCa), and the CT-X antigen prostate-associated gene 4 (PAGE4) exhibited augmented expression in primary PCa [82] Correspondingly, MAGE-C2/CT10 appeared as a marker for relapsing prostate cancer post-radical prostatectomy, evident in around 3% of primary PCa cases only [83]. These findings indicated the possibility of developing CTA-based “gene signature” for prostate cancer patients. It also underscored the need for multi-functionality as a key characteristic of CTAs, which may serve as potential biomarker candidates [75]. A PCR-based assay, involving a list of 22 CTAs on a high-risk cohort with advanced tumorous prostate cancer, demonstrated that the non-X CTAs increased, while the CT-X antigen, PAGE4, decreased in the aggressive form of PCa, following prostate gland and associated tissue removal. The study pointed to the stage specific expression of CTAs in prostate cancer [84]. Corroborating this, a sensitive, consistent, descriptive, integrated and highly intricate nCounter Analysis System through mRNA-based assay identified CEP55, NUF2, TTK, PDZ-Binding Kinase (PBK) and PAGE4 as undergoing distinctive expression in benign, primary, non-metastatic and metastatic forms of prostate cancer [85].
CTAs served as prominent participants in the process of cell cycle progression and cell proliferation. Notably, MAGEC2-specific immunotherapies removed malignant myeloma tumors. Here MAGEC2 silencing suppressed the cell number in S phase, inhibited G0/G1 and G2/M and enhanced apoptotic cell death via enhanced cell population in sub-G0/G1 diploid phase [86]. Sustaining the characteristics of an inherently chaotic protein, PAGE4 functions as a mutated oncogene via enhanced activity of Activator Protein-1 transcription factor and phosphorylation, involving the homeodomain-interacting protein kinase-1 and CDC-like kinase-2 [87]. Hence, PAGE4 shows normal expression in benign prostate glands and an aberrant expression in androgen-dependent and non-androgen-independent prostate cancers. In fact, PAGE4-AP-1-AR-CLK2 pathway drives the androgen-dependent and -independent conditions in prostate cancers [88]. Hence, phosphorylation and the shift between androgenicity and non-androgenicity confer PAGE4 sensitivity to prostate cancer, overall, implying its potential as a novel biomarker and therapeutic target for prostate cancer. These observations highlight the functional relevance of CTAs as biomarkers in specifying status of the disease, as well as inducing a balanced cell cycle regulation, equilibrium and cellular protection. An additional advantage of PAGE4 as a marker for prostate cancer stems from the fact that unlike the serum prostate serum antigen (PSA), PAGE4 is undetectable in the normal prostate glands. PAGE4 plays a prominent role in distinguishing different types and grades of benign prostate hyperplasia that associate with chronic bladder obstruction, urinary retention, renal failure, persistent urinary tract infections, renal bleeding and urinary tract and kidney stones [87]. Hence, regulated, PAGE4 expression represents a novel strategy for generating personalized medicine against prostate cancer. The serum PAGE4 levels also help discerning the androgen receptor-reliant castration-resistant prostate cancer and metastasis and androgen receptor-independent forms of prostate cancers. The phospho-PAGE4 interacts with AP-1, triggering an androgen receptor-mediated mechanism, which functions as a critical therapeutic target for prostate cancer and metastasis [89]. The androgen receptor-independent form primarily involves glucocorticoid receptor signaling and tumoral immune resistance, with the participation of neuroendocrine cell population that influences prostate cancer development and growth. Because the shift between phosphorylated and non-phosphorylated forms of PAGE4 plays a key role in the prostate cancer fate, monoclonal antibodies against the diverse phospho-PAGE4 forms and RNA-Seq methods based on PAGE4 RNA help detecting the different stages of prostate cancer progression [76,90]. Hence, differential PAGE4 phosphorylation and its link with androgen dependence regulate the phenotypic heterogeneity of prostate cancer [87]. Unlike kallikrein proteases, PAGE4 is an enriched and intrinsically disordered protein that bears versatile conformational dynamics for binding with a wide-range of proteins. The conformational ensembles are more prominent in malignant rather than benign conditions, bearing disparate sensitivities to therapies [91,92]. Additionally, conformational plasticity of the intrinsically disordered protein forms of PAGE4 proto-oncogene leads to its different mutated forms at diverse stages and conditions during prostate cancer progression [93].
Cervical cancer and HNSCC involve an abnormal methylation of CpG islands, indicating epigenetic changes. Moreover, the Nucleolar Protein 4 (NOL4) gene promoter associates with about 80-90% of cervical and HNSCC [94]. Alternate splicing plays a key role in biological functionality of NOL4, causing discrete regulation of the downstream transcription factors that control varied cell specific activities [75,95,96]. The post-translational modification of NOL4 in association with alternate splicing guide site-specific molecular interaction and nuclear localization signals that affect cervical cancer and HNSCC conditions [75].
CEP55 is normally present in the testis and thymus, but is detected in significant amounts in the tissues of breast carcinoma, colorectal, lung, colon and bladder cancers. CEP55 plays an important role in cell fate determination in breast cancer and functions as a prognostic in NSCLC. It associates with the clinicopathological features of bladder cancer, determines the sensitivity of colon cancer to T cell responses and appears as common methylation clusters in colorectal cancer [97-101]. CEP55 serves as a biomarker for estrogen-responsive breast cancer and HNSCC, where it facilitates cancer development via abnormal triggering of the Forkhead box proto-oncogene family of transcription factors [102,103]. In intestinal cancer, CEP55 increases the physical process of cell division via an intricate pathway involving the tumorigenic peptidyl-prolyl cis/trans-isomerase (Pin1) binding to inhibitory Polo-like kinase 1 (Plk1) in cellular mitosis and proliferative AKT1 signaling pathway. A deficiency or mutation in tumor suppressor, BRCA2 (DNA repair associated), deregulates the normal CEP55 complexation with the apoptotic proteins that promote cytokinetic abscission [104]. The process also entails alternative splicing and reduced kinase activity or removal of phospho-domains and molecular recognition features that alternate between ordered and disordered conditions, while interacting with their suitable stipulated proteins. Hence, distinct protein isoform products of alternate RNA splicing and posttranslational modification of NOL4, PAGE4 and CEP55, which induce highly dynamic conformational ensembles and functional changeability in the CTA proteins, designate them as next generation biomarker candidates for the different stages and forms of cancer [75].
Structural plasticity and multifunctionality of CTAs have rendered them as appropriate biomarker candidates for detection and confirmation of cancers. CTAs interact with DNA, induce epigenetic changes, cell proliferation and cell cycle progression and alter protein regulation, affecting the process of carcinogenesis. Nonetheless, wide clinical studies are essential for considering CTAs as ideal biomarkers for discerning stage-specific prognosis in diverse forms of cancer.
CTAs, tumor vaccine and immunotherapy
Tumor immunotherapy has advanced significantly with the development of hybridoma technology and antigen processing and presentation. Discovery of the strongly immunogenic CTAs contributed to tumor vaccine development, having been considered as perfect targets for immunotherapy. CTA peptides and clinical trials with the CTA multipeptide vaccines have led to the way for novel personalized CTA peptide vaccines [105]. CTAs have been regarded as perfect targets for cancer vaccines, owing to their significant expression in various humor tumors compared to benign cells [106]. CTA-based vaccine treatment appeared very helpful in breast cancer patients with otherwise fewer options for therapy, especially those with triple-negative breast cancer [5]. CTAs are important for the testicles that fail to mount immune responses and are devoid of adaptive immunity and blood-testis barrier that sequester germ cells [22]. They offer an immune-privileged microenvironment, towards completing the process of meiosis [33]. The CTA genes are abundant in the testis, and participate in meiosis during spermatogenesis and tumorigenesis having shared features [107]. Owing to their extremely systematic and precise characteristics, CTAs function as biomarkers for early detection of cancer and are suitable for immunotherapeutic approaches. It has also been seen that patients failing to respond to the first- and second-line cancer therapies could benefit from CTA-dependent immunotherapy [105]. CTAs also contributed significantly to cancer vaccination and adoptive transfer of the chimeric receptor-based peripheral blood-derived T lymphocytes [108]. The immune responses usually involved induction of effector and memory CD8+ (cytotoxic) and CD4+ Helper T cells in the tumor micro-environment, with the activation of tumor specific antigens [109,110]. For the last few years, progress in the domain of CTA as a mode of immunotherapy has led to its importance as replacement for conventional cancer treatment [26,111,112]. Accordingly, a marked effort towards developing proficiency and cautiousness for the generation and application of the ideal CTA (to avoid the risk of metastasis) for clinical testing in cancer is currently underway [26]. CTAs have been reckoned for protective cancer immunotherapy, owing to their extensive role in attenuating tumor [105]. Hence, a broad range of peptides, proteins, DNA, RNA and viral and bacterial vector-based vaccine strategies with distinct affinity for CTAs is under development and considered for different clinical trials [106].
Generally, clinical trials in melanoma patients showed involvement of dendritic cell-dependent vaccines or human leukocyte antigen-based antigenic determinants in relation to MAGE-A1- and MAGE-A3-positive cancer types. The Phase I trial on vaccination demonstrated a prominent increase in the CD8+ antigen-specific T-cell clones targeting MAGE-1, GP100 peptide vaccine and human epidermal growth factor receptor-2 [113,114]. MAGE-3 exhibited spontaneous immune responses in cancer patients, and hence, immunization with MAGE-A3 and clinical trials with MAGE-A3 vaccines laid claim to its use in cancer immunotherapy [115]. NY-ESO-1-based patient studies involved the human leukocyte antigen-specific antigenic determinants, recognized by the immune system or total protein. The treatment often included an adjuvant that suppressed secondary tumor formation, post-vaccination. The adjuvant treatment promoted antibody-mediated cellular immunity, generally used at several doses and mixtures, targeting dendritic cells in patients with NY-ESO-1-marked tumors [116,117]. The therapeutic process involved an antigen-specific immune response, with prominent CD8+ and CD4+ T cell infiltration. The NY-ESO-based peptides together with adjuvants stimulated prominent CD8+ and CD4+ T-cell immune reactions in melanoma patients, validating the potential of the immunotherapeutic strategy [118]. Patients treated with whole protein and/or adjuvant showed diverse immune responses, and varied reactions are also detected in clinical cases showing cancer relapse [106].
A combination of CTA-based therapy and chemotherapeutic agent, like decitabine, that causes epigenetic stimulation has been considered as a major strategy for tumor therapy. Via suppressed hypermethylation (that inhibited CTA expression) and enhanced demethylation, decitabine stimulates MAGE-A1, MAGE-A3 and NY-ESO-1 genes in malignant cells in a number of tumor cell lines, such as, esophageal carcinoma, malignant melanoma, glioma, etc. [119,120]. Decitabine, plays an important role in promoting clinical potential of MAGE-A3, MAGE-A1 and NY-ESO-1-specific therapy by up-regulating transcription of essential target genes [119,120]. CTA over-expression and treatment with decitabine successfully leads to the death of tumorous cells, and the combination serves as a key anti-tumor strategy for treating patients with recurring cancer and metastasis [106,114,121,122]. Moreover, MAGE-A1, MAGE-A3 and NY-ESO-1-specific T cells in combination with decitabine helped in the expansion of Cancer Germline antigen-Specific Cytotoxic T Lymphocytes for vaccine and immunotherapy [123]. An expansion in the tumor reactive T cells for adoptive immunotherapy in combination with CTA-based vaccine therapy is a potential immunotherapeutic strategy for cancer. This alters tumor immune invasion and enhances the tumor-reactive T cell count. The CTAs are appropriate targets for high tumor-specificity and induction of humoral and cell-mediated immune responses, and a combination of vaccination and adoptive transfer serves the purpose [124]. While vaccines together with adjuvants increase the inherent immune responsive property of CTA, CTA-based T cell expansion for adoptive transfer has been hypothesized as a prospective procedure for reducing the risk of cancer metastasis (Figure 3).
Figure 3.
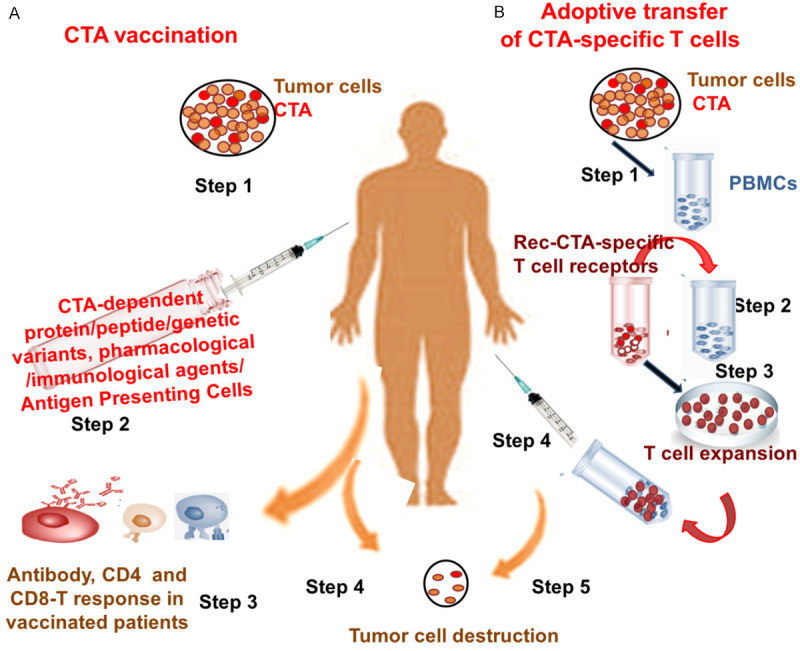
CTA in Cancer Immunotherapy. CTA vaccination (A) and strategy involving Adoptive transfer of CTA-specific T cells (B) induce immunity & cancer therapy. CTA vaccination (A), Step 1: Detection of CTAs in Tumors; Step 2: Treating patient with CTA-dependent protein/peptide/genetic variant along with pharmacological or immunological agents or Antigen Presenting Cells; Step 3: CD4, CD8 T cell and antibody response in vaccinated patient; Step 4: Spontaneous immunity in patient with advanced malignancy & metastasis. Adoptive transfer of CTA-specific T cells (B), Step 1: Detection of CTAs in Tumors and isolation of peripheral blood mononuclear cells (PBMCs); Step 2: Transfer Recombinant specific T cell receptors to PBMCs; Step 3: Expansion of T cells; Step 4: T cell treatment in patient; Step 5: Spontaneous immunity in patients with advanced malignancy & metastasis.
Conclusion
Identification of CTA-expressing cancer cells is a key factor in CTA-dependent vaccine and immunotherapy, particularly against the wide-spread epithelial cancers. The CTAs that perfectly exhibit these immunotherapeutic functions need to be target-specific, expressed in abundance in tumor and rarely in normal cells, and stably and consistently present in the malignant cells. CTAs are expected to have defined functional characterization and oncogenicity, capable of attenuating tumorigenic potential and reducing chances of metastasis. Nonetheless, hardly any CTA yet satisfies these ideal features, and hence CTA-mediated clinical trials appear as a distant fact. Combination therapy of CTA with decitabine and chemotherapy may partially solve the problem; however, exclusive use of CTA for the purpose needs severe investigation and thorough research. Moreover, specific targets, appropriate clinical settings for patient trials and availability of suitable combination therapies are challenges in the field of CTA peptide-based vaccines for cancer immunotherapy. Notably, it has been predicted that CTA peptides in association with immunogenic adjuvants and adoptive transfer of T cells may develop as personalized vaccines, towards treating malignant tumors individually and an ultimate patient survival.
Disclosure of conflict of interest
None.
References
- 1.Fratta E, Coral S, Covre A, Parisi G, Colizzi F, Danielli R, Nicolay HJ, Sigalotti L, Maio M. The biology of cancer testis antigens: putative function, regulation and therapeutic potential. Mol Oncol. 2011;5:164–182. doi: 10.1016/j.molonc.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei R, Dean DC, Thanindratarn P, Hornicek FJ, Guo W, Duan Z. Cancer testis antigens in sarcoma: expression, function and immunotherapeutic application. Cancer Lett. 2020;479:54–60. doi: 10.1016/j.canlet.2019.10.024. [DOI] [PubMed] [Google Scholar]
- 3.Inoda S, Morita R, Hirohashi Y, Torigoe T, Asanuma H, Nakazawa E, Nakatsugawa M, Tamura Y, Kamiguchi K, Tsuruma T, Terui T, Ishitani K, Hashino S, Wang Q, Greene MI, Hasegawa T, Hirata K, Asaka M, Sato N. The feasibility of Cep55/c10orf3 derived peptide vaccine therapy for colorectal carcinoma. Exp Mol Pathol. 2011;90:55–60. doi: 10.1016/j.yexmp.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahmoud AM. Cancer testis antigens as immunogenic and oncogenic targets in breast cancer. Immunotherapy. 2018;10:769–778. doi: 10.2217/imt-2017-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Li M. Correlate tumor mutation burden with immune signatures in human cancers. BMC Immunol. 2019;20:4. doi: 10.1186/s12865-018-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babatunde KA, Najafi A, Salehipour P, Modarressi MH, Mobasheri MB. Cancer/Testis genes in relation to sperm biology and function. Iran J Basic Med Sci. 2017;20:967–974. doi: 10.22038/IJBMS.2017.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Safavi A, Kefayat A, Ghahremani F, Mahdevar E, Moshtaghian J. Immunization using male germ cells and gametes as rich sources of cancer/testis antigens for inhibition of 4T1 breast tumors’ growth and metastasis in BALB/c mice. Int Immunopharmacol. 2019;74:105719. doi: 10.1016/j.intimp.2019.105719. [DOI] [PubMed] [Google Scholar]
- 9.Jones PA, Ohtani H, Chakravarthy A, De Carvalho DD. Epigenetic therapy in immune-oncology. Nat Rev Cancer. 2019;19:151–161. doi: 10.1038/s41568-019-0109-9. [DOI] [PubMed] [Google Scholar]
- 10.Xie K, Fu C, Wang S, Xu H, Liu S, Shao Y, Gong Z, Wu X, Xu B, Han J, Xu J, Xu P, Jia X, Wu J. Cancer-testis antigens in ovarian cancer: implication for biomarkers and therapeutic targets. J Ovarian Res. 2019;12:1. doi: 10.1186/s13048-018-0475-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen F, Zhang Y, Parra E, Rodriguez J, Behrens C, Akbani R, Lu Y, Kurie JM, Gibbons DL, Mills GB, Wistuba II, Creighton CJ. Multiplatform-based molecular subtypes of non-small-cell lung cancer. Oncogene. 2017;36:1384–1393. doi: 10.1038/onc.2016.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Chu J, Li J, Feng W, Yang F, Wang Y, Zhang Y, Sun C, Yang M, Vasilatos SN, Huang Y, Fu Z, Yin Y. Cancer/testis antigen-Plac1 promotes invasion and metastasis of breast cancer through Furin/NICD/PTEN signaling pathway. Mol Oncol. 2018;12:1233–1248. doi: 10.1002/1878-0261.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Arcy P, Maruwge W, Wolahan B, Ma L, Brodin B. Oncogenic functions of the cancer-testis antigen SSX on the proliferation, survival, and signaling pathways of cancer cells. PLoS One. 2014;9:e95136. doi: 10.1371/journal.pone.0095136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y, Zou R, Wang J, Wang ZW, Zhu X. The role of the cancer testis antigen PRAME in tumorigenesis and immunotherapy in human cancer. Cell Prolif. 2020;53:e12770. doi: 10.1111/cpr.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao L, Lanz RB, Frolov A, Castro PD, Zhang Z, Dong B, Xue W, Jung SY, Lydon JP, Edwards DP, Mancini MA, Feng Q, Ittmann MM, He B. The germ cell gene TDRD1 as an ERG target gene and a novel prostate cancer biomarker. Prostate. 2016;76:1271–1284. doi: 10.1002/pros.23213. [DOI] [PubMed] [Google Scholar]
- 16.Aoki N, Matsui Y. Comprehensive analysis of mouse cancer/testis antigen functions in cancer cells and roles of TEKT5 in cancer cells and testicular germ cells. Mol Cell Biol. 2019;39:e00154-19. doi: 10.1128/MCB.00154-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibbs ZA, Whitehurst AW. Emerging contributions of cancer/testis antigens to neoplastic behaviors. Trends Cancer. 2018;4:701–712. doi: 10.1016/j.trecan.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Z, Zuo X, Pu L, Zhang Y, Han G, Zhang L, Wu Z, You W, Qin J, Dai X, Shen H, Wang X, Wu J. Hypomethylation-mediated activation of cancer/testis antigen KK-LC-1 facilitates hepatocellular carcinoma progression through activating the Notch1/Hes1 signalling. Cell Prolif. 2019;52:e12581. doi: 10.1111/cpr.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W, Barger CJ, Link PA, Mhawech-Fauceglia P, Miller A, Akers SN, Odunsi K, Karpf AR. DNA hypomethylation-mediated activation of Cancer/Testis Antigen 45 (CT45) genes is associated with disease progression and reduced survival in epithelial ovarian cancer. Epigenetics. 2015;10:736–748. doi: 10.1080/15592294.2015.1062206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kew M. Alpha-fetoprotein in primary liver cancer and other diseases. Gut. 1974;15:814–821. doi: 10.1136/gut.15.10.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke P, Mann J, Simpson JF, Rickard-Dickson K, Primus FJ. Mice transgenic for human carcinoembryonic antigen as a model for immunotherapy. Cancer Res. 1998;58:1469–1477. [PubMed] [Google Scholar]
- 22.Kalejs M, Erenpreisa J. Cancer/testis antigens and gametogenesis: a review and “brain-storming” session. Cancer Cell Int. 2005;5:4. doi: 10.1186/1475-2867-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abelev GI, Perova SD, Khramkova NI, Postnikova ZA, Irlin IS. Production of embryonal alpha-globulin by transplantable mouse hepatomas. Transplantation. 1963;1:174–180. doi: 10.1097/00007890-196301020-00004. [DOI] [PubMed] [Google Scholar]
- 24.Bodey B, Siegel SE, Kaiser HE. MAGE-1, a cancer/testis-antigen, expression in childhood astrocytomas as an indicator of tumor progression. In Vivo. 2002;16:583–588. [PubMed] [Google Scholar]
- 25.Liu C, Luo B, Xie XX, Liao XS, Fu J, Ge YY, Li XS, Guo GS, Shen N, Xiao SW, Zhang QM. Involvement of X-chromosome reactivation in augmenting cancer testis antigens expression: a hypothesis. Curr Med Sci. 2018;38:19–25. doi: 10.1007/s11596-018-1842-0. [DOI] [PubMed] [Google Scholar]
- 26.Salmaninejad A, Zamani MR, Pourvahedi M, Golchehre Z, Hosseini Bereshneh A, Rezaei N. Cancer/testis antigens: expression, regulation, tumor invasion, and use in immunotherapy of cancers. Immunol Invest. 2016;45:619–640. doi: 10.1080/08820139.2016.1197241. [DOI] [PubMed] [Google Scholar]
- 27.Chen YT, Chiu R, Lee P, Beneck D, Jin B, Old LJ. Chromosome X-encoded cancer/testis antigens show distinctive expression patterns in developing gonads and in testicular seminoma. Hum Reprod. 2011;26:3232–3243. doi: 10.1093/humrep/der330. [DOI] [PubMed] [Google Scholar]
- 28.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 29.Fon Tacer K, Montoya MC, Oatley MJ, Lord T, Oatley JM, Klein J, Ravichandran R, Tillman H, Kim M, Connelly JP, Pruett-Miller SM, Bookout AL, Binshtock E, Kaminski MM, Potts PR. MAGE cancer-testis antigens protect the mammalian germline under environmental stress. Sci Adv. 2019;5:eaav4832. doi: 10.1126/sciadv.aav4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jungbluth AA, Antonescu CR, Busam KJ, Iversen K, Kolb D, Coplan K, Chen YT, Stockert E, Ladanyi M, Old LJ. Monophasic and biphasic synovial sarcomas abundantly express cancer/testis antigen NY-ESO-1 but not MAGE-A1 or CT7. Int J Cancer. 2001;94:252–256. doi: 10.1002/ijc.1451. [DOI] [PubMed] [Google Scholar]
- 31.Caballero OL, Chen YT. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci. 2009;100:2014–2021. doi: 10.1111/j.1349-7006.2009.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeda K, Kitaura K, Suzuki R, Owada Y, Muto S, Okabe N, Hasegawa T, Osugi J, Hoshino M, Tsunoda T, Okumura K, Suzuki H. Quantitative T-cell repertoire analysis of peripheral blood mononuclear cells from lung cancer patients following long-term cancer peptide vaccination. Cancer Immunol Immunother. 2018;67:949–964. doi: 10.1007/s00262-018-2152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng YH, Wong EW, Cheng CY. Cancer/testis (CT) antigens, carcinogenesis and spermatogenesis. Spermatogenesis. 2011;1:209–220. doi: 10.4161/spmg.1.3.17990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doyle JM, Gao J, Wang J, Yang M, Potts PR. MAGE-RING protein complexes comprise a family of E3 ubiquitin ligases. Mol Cell. 2010;39:963–974. doi: 10.1016/j.molcel.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schutt CA, Mirandola L, Figueroa JA, Nguyen DD, Cordero J, Bumm K, Judson BL, Chiriva-Internati M. The cancer-testis antigen, sperm protein 17, a new biomarker and immunological target in head and neck squamous cell carcinoma. Oncotarget. 2017;8:100280–100287. doi: 10.18632/oncotarget.22213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weon JL, Potts PR. The MAGE protein family and cancer. Curr Opin Cell Biol. 2015;37:1–8. doi: 10.1016/j.ceb.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu WS. Mammalian sex chromosome structure, gene content, and function in male fertility. Annu Rev Anim Biosci. 2019;7:103–124. doi: 10.1146/annurev-animal-020518-115332. [DOI] [PubMed] [Google Scholar]
- 38.Thomas R, Al-Khadairi G, Roelands J, Hendrickx W, Dermime S, Bedognetti D, Decock J. NY-ESO-1 based immunotherapy of cancer: current perspectives. Front Immunol. 2018;9:947. doi: 10.3389/fimmu.2018.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esfandiary A, Ghafouri-Fard S. New York esophageal squamous cell carcinoma-1 and cancer immunotherapy. Immunotherapy. 2015;7:411–439. doi: 10.2217/imt.15.3. [DOI] [PubMed] [Google Scholar]
- 40.Zhang T, Zhou Y, Qi ST, Wang ZB, Qian WP, Ouyang YC, Shen W, Schatten H, Sun QY. Nuf2 is required for chromosome segregation during mouse oocyte meiotic maturation. Cell Cycle. 2015;14:2701–2710. doi: 10.1080/15384101.2015.1058677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinha D, Kalimutho M, Bowles J, Chan AL, Merriner DJ, Bain AL, Simmons JL, Freire R, Lopez JA, Hobbs RM, O’Bryan MK, Khanna KK. Cep55 overexpression causes male-specific sterility in mice by suppressing Foxo1 nuclear retention through sustained activation of PI3K/Akt signaling. FASEB J. 2018;32:4984–4999. doi: 10.1096/fj.201701096RR. [DOI] [PubMed] [Google Scholar]
- 42.Choi H, Jin S, Kwon JT, Kim J, Jeong J, Kim J, Jeon S, Park ZY, Jung KJ, Park K, Cho C. Characterization of mammalian ADAM2 and its absence from human sperm. PLoS One. 2016;11:e0158321. doi: 10.1371/journal.pone.0158321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghafouri-Fard S, Modarressi MH. Cancer-testis antigens: potential targets for cancer immunotherapy. Arch Iran Med. 2009;12:395–404. [PubMed] [Google Scholar]
- 44.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 45.Yao J, Caballero OL, Yung WK, Weinstein JN, Riggins GJ, Strausberg RL, Zhao Q. Tumor subtype-specific cancer-testis antigens as potential biomarkers and immunotherapeutic targets for cancers. Cancer Immunol Res. 2014;2:371–379. doi: 10.1158/2326-6066.CIR-13-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radich JP, Dai H, Mao M, Oehler V, Schelter J, Druker B, Sawyers C, Shah N, Stock W, Willman CL, Friend S, Linsley PS. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc Natl Acad Sci U S A. 2006;103:2794–2799. doi: 10.1073/pnas.0510423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tio D, Kasiem FR, Willemsen M, van Doorn R, van der Werf N, Hoekzema R, Luiten RM, Bekkenk MW. Expression of cancer/testis antigens in cutaneous melanoma: a systematic review. Melanoma Res. 2019;29:349–357. doi: 10.1097/CMR.0000000000000569. [DOI] [PubMed] [Google Scholar]
- 48.Hermes N, Kewitz S, Staege MS. Preferentially expressed antigen in melanoma (PRAME) and the PRAME family of leucine-rich repeat proteins. Curr Cancer Drug Targets. 2016;16:400–414. doi: 10.2174/1568009616666151222151818. [DOI] [PubMed] [Google Scholar]
- 49.Wang D, Wang J, Ding N, Li Y, Yang Y, Fang X, Zhao H. MAGE-A1 promotes melanoma proliferation and migration through C-JUN activation. Biochem Biophys Res Commun. 2016;473:959–965. doi: 10.1016/j.bbrc.2016.03.161. [DOI] [PubMed] [Google Scholar]
- 50.Tung KS, Harakal J, Qiao H, Rival C, Li JC, Paul AG, Wheeler K, Pramoonjago P, Grafer CM, Sun W, Sampson RD, Wong EW, Reddi PP, Deshmukh US, Hardy DM, Tang H, Cheng CY, Goldberg E. Egress of sperm autoantigen from seminiferous tubules maintains systemic tolerance. J Clin Invest. 2017;127:1046–1060. doi: 10.1172/JCI89927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiappinelli KB, Zahnow CA, Ahuja N, Baylin SB. Combining epigenetic and immunotherapy to combat cancer. Cancer Res. 2016;76:1683–1689. doi: 10.1158/0008-5472.CAN-15-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akers SN, Odunsi K, Karpf AR. Regulation of cancer germline antigen gene expression: implications for cancer immunotherapy. Future Oncol. 2010;6:717–732. doi: 10.2217/fon.10.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karpf AR. A potential role for epigenetic modulatory drugs in the enhancement of cancer/germ-line antigen vaccine efficacy. Epigenetics. 2006;1:116–120. doi: 10.4161/epi.1.3.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeng G, Touloukian CE, Wang X, Restifo NP, Rosenberg SA, Wang RF. Identification of CD4+ T cell epitopes from NY-ESO-1 presented by HLA-DR molecules. J Immunol. 2000;165:1153–1159. doi: 10.4049/jimmunol.165.2.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Wang Z, Zhang J, Lim SH. Core promoter sequence of SEMG I spans between the two putative GATA-1 binding domains and is responsive to IL-4 and IL-6 in myeloma cells. Leuk Res. 2009;33:166–169. doi: 10.1016/j.leukres.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karpf AR, Lasek AW, Ririe TO, Hanks AN, Grossman D, Jones DA. Limited gene activation in tumor and normal epithelial cells treated with the DNA methyltransferase inhibitor 5-aza-2’-deoxycytidine. Mol Pharmacol. 2004;65:18–27. doi: 10.1124/mol.65.1.18. [DOI] [PubMed] [Google Scholar]
- 57.Roman-Gomez J, Jimenez-Velasco A, Agirre X, Castillejo JA, Navarro G, San Jose-Eneriz E, Garate L, Cordeu L, Cervantes F, Prosper F, Heiniger A, Torres A. Epigenetic regulation of human cancer/testis antigen gene, HAGE, in chronic myeloid leukemia. Haematologica. 2007;92:153–162. doi: 10.3324/haematol.10782. [DOI] [PubMed] [Google Scholar]
- 58.Sun AG, Wang MG, Li B, Meng FG. Down-regulation of miR-124 target protein SCP-1 inhibits neuroglioma cell migration. Eur Rev Med Pharmacol Sci. 2017;21:723–729. [PubMed] [Google Scholar]
- 59.Lee JH, Schutte D, Wulf G, Fuzesi L, Radzun HJ, Schweyer S, Engel W, Nayernia K. Stem-cell protein Piwil2 is widely expressed in tumors and inhibits apoptosis through activation of Stat3/Bcl-XL pathway. Hum Mol Genet. 2006;15:201–211. doi: 10.1093/hmg/ddi430. [DOI] [PubMed] [Google Scholar]
- 60.Litwin M, Szczepanska-Buda A, Piotrowska A, Dziegiel P, Witkiewicz W. The meaning of PIWI proteins in cancer development. Oncol Lett. 2017;13:3354–3362. doi: 10.3892/ol.2017.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sheu-Gruttadauria J, MacRae IJ. Structural foundations of RNA silencing by argonaute. J Mol Biol. 2017;429:2619–2639. doi: 10.1016/j.jmb.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X, Lv C, Guo Y, Yuan S. Mitochondria associated germinal structures in spermatogenesis: pirna pathway regulation and beyond. Cells. 2020;9:399. doi: 10.3390/cells9020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suzuki R, Honda S, Kirino Y. PIWI expression and function in cancer. Front Genet. 2012;3:204. doi: 10.3389/fgene.2012.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malone CD, Brennecke J, Dus M, Stark A, McCombie WR, Sachidanandam R, Hannon GJ. Specialized piRNA pathways act in germline and somatic tissues of the Drosophila ovary. Cell. 2009;137:522–535. doi: 10.1016/j.cell.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Greve KB, Lindgreen JN, Terp MG, Pedersen CB, Schmidt S, Mollenhauer J, Kristensen SB, Andersen RS, Relster MM, Ditzel HJ, Gjerstorff MF. Ectopic expression of cancer/testis antigen SSX2 induces DNA damage and promotes genomic instability. Mol Oncol. 2015;9:437–449. doi: 10.1016/j.molonc.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Asquith KL, Harman AJ, McLaughlin EA, Nixon B, Aitken RJ. Localization and significance of molecular chaperones, heat shock protein 1, and tumor rejection antigen gp96 in the male reproductive tract and during capacitation and acrosome reaction. Biol Reprod. 2005;72:328–337. doi: 10.1095/biolreprod.104.034470. [DOI] [PubMed] [Google Scholar]
- 67.Gleason RJ, Anand A, Kai T, Chen X. Protecting and diversifying the germline. Genetics. 2018;208:435–471. doi: 10.1534/genetics.117.300208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jasti S, Farahbakhsh M, Nguyen S, Petroff BK, Petroff MG. Immune response to a model shared placenta/tumor-associated antigen reduces cancer risk in parous mice. Biol Reprod. 2017;96:134–144. doi: 10.1095/biolreprod.116.144907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Almstedt M, Blagitko-Dorfs N, Duque-Afonso J, Karbach J, Pfeifer D, Jager E, Lubbert M. The DNA demethylating agent 5-aza-2’-deoxycytidine induces expression of NY-ESO-1 and other cancer/testis antigens in myeloid leukemia cells. Leuk Res. 2010;34:899–905. doi: 10.1016/j.leukres.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 70.Heninger E, Krueger TE, Thiede SM, Sperger JM, Byers BL, Kircher MR, Kosoff D, Yang B, Jarrard DF, McNeel DG, Lang JM. Inducible expression of cancer-testis antigens in human prostate cancer. Oncotarget. 2016;7:84359–84374. doi: 10.18632/oncotarget.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim R, Kulkarni P, Hannenhalli S. Derepression of Cancer/testis antigens in cancer is associated with distinct patterns of DNA hypomethylation. BMC Cancer. 2013;13:144. doi: 10.1186/1471-2407-13-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kubota H, Brinster RL. Spermatogonial stem cells. Biol Reprod. 2018;99:52–74. doi: 10.1093/biolre/ioy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Griswold MD. Spermatogenesis: the commitment to meiosis. Physiol Rev. 2016;96:1–17. doi: 10.1152/physrev.00013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suri A. Cancer testis antigens--their importance in immunotherapy and in the early detection of cancer. Expert Opin Biol Ther. 2006;6:379–389. doi: 10.1517/14712598.6.4.379. [DOI] [PubMed] [Google Scholar]
- 75.Kulkarni P, Uversky VN. Cancer/Testis Antigens: “Smart” biomarkers for diagnosis and prognosis of prostate and other cancers. Int J Mol Sci. 2017;18:740. doi: 10.3390/ijms18040740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He Y, Chen Y, Mooney SM, Rajagopalan K, Bhargava A, Sacho E, Weninger K, Bryan PN, Kulkarni P, Orban J. Phosphorylation-induced conformational ensemble switching in an intrinsically disordered cancer/testis antigen. J Biol Chem. 2015;290:25090–25102. doi: 10.1074/jbc.M115.658583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Velazquez EF, Jungbluth AA, Yancovitz M, Gnjatic S, Adams S, O’Neill D, Zavilevich K, Albukh T, Christos P, Mazumdar M, Pavlick A, Polsky D, Shapiro R, Berman R, Spira J, Busam K, Osman I, Bhardwaj N. Expression of the cancer/testis antigen NY-ESO-1 in primary and metastatic malignant melanoma (MM)--correlation with prognostic factors. Cancer Immun. 2007;7:11. [PMC free article] [PubMed] [Google Scholar]
- 78.Saxena M, Bhardwaj N. Turbocharging vaccines: emerging adjuvants for dendritic cell based therapeutic cancer vaccines. Curr Opin Immunol. 2017;47:35–43. doi: 10.1016/j.coi.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hasegawa K, Noguchi Y, Koizumi F, Uenaka A, Tanaka M, Shimono M, Nakamura H, Shiku H, Gnjatic S, Murphy R, Hiramatsu Y, Old LJ, Nakayama E. In vitro stimulation of CD8 and CD4 T cells by dendritic cells loaded with a complex of cholesterol-bearing hydrophobized pullulan and NY-ESO-1 protein: identification of a new HLA-DR15-binding CD4 T-cell epitope. Clin Cancer Res. 2006;12:1921–1927. doi: 10.1158/1078-0432.CCR-05-1900. [DOI] [PubMed] [Google Scholar]
- 80.Cronwright G, Le Blanc K, Gotherstrom C, Darcy P, Ehnman M, Brodin B. Cancer/testis antigen expression in human mesenchymal stem cells: down-regulation of SSX impairs cell migration and matrix metalloproteinase 2 expression. Cancer Res. 2005;65:2207–2215. doi: 10.1158/0008-5472.CAN-04-1882. [DOI] [PubMed] [Google Scholar]
- 81.Yang P, Huo Z, Liao H, Zhou Q. Cancer/testis antigens trigger epithelial-mesenchymal transition and genesis of cancer stem-like cells. Curr Pharm Des. 2015;21:1292–1300. doi: 10.2174/1381612821666141211154707. [DOI] [PubMed] [Google Scholar]
- 82.Suyama T, Shiraishi T, Zeng Y, Yu W, Parekh N, Vessella RL, Luo J, Getzenberg RH, Kulkarni P. Expression of cancer/testis antigens in prostate cancer is associated with disease progression. Prostate. 2010;70:1778–1787. doi: 10.1002/pros.21214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.von Boehmer L, Keller L, Mortezavi A, Provenzano M, Sais G, Hermanns T, Sulser T, Jungbluth AA, Old LJ, Kristiansen G, van den Broek M, Moch H, Knuth A, Wild PJ. MAGE-C2/CT10 protein expression is an independent predictor of recurrence in prostate cancer. PLoS One. 2011;6:e21366. doi: 10.1371/journal.pone.0021366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shiraishi T, Getzenberg RH, Kulkarni P. Cancer/testis antigens: novel tools for discerning aggressive and non-aggressive prostate cancer. Asian J Androl. 2012;14:400–404. doi: 10.1038/aja.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kulkarni P, Shiraishi T, Rajagopalan K, Kim R, Mooney SM, Getzenberg RH. Cancer/testis antigens and urological malignancies. Nat Rev Urol. 2012;9:386–396. doi: 10.1038/nrurol.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lajmi N, Luetkens T, Yousef S, Templin J, Cao Y, Hildebrandt Y, Bartels K, Kroger N, Atanackovic D. Cancer-testis antigen MAGEC2 promotes proliferation and resistance to apoptosis in Multiple Myeloma. Br J Haematol. 2015;171:752–762. doi: 10.1111/bjh.13762. [DOI] [PubMed] [Google Scholar]
- 87.Salgia R, Jolly MK, Dorff T, Lau C, Weninger K, Orban J, Kulkarni P. Prostate-associated gene 4 (PAGE4): leveraging the conformational dynamics of a dancing protein cloud as a therapeutic target. J Clin Med. 2018;7:156. doi: 10.3390/jcm7060156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kulkarni P, Jolly MK, Jia D, Mooney SM, Bhargava A, Kagohara LT, Chen Y, Hao P, He Y, Veltri RW, Grishaev A, Weninger K, Levine H, Orban J. Phosphorylation-induced conformational dynamics in an intrinsically disordered protein and potential role in phenotypic heterogeneity. Proc Natl Acad Sci U S A. 2017;114:E2644–E2653. doi: 10.1073/pnas.1700082114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin X, Roy S, Jolly MK, Bocci F, Schafer NP, Tsai MY, Chen Y, He Y, Grishaev A, Weninger K, Orban J, Kulkarni P, Rangarajan G, Levine H, Onuchic JN. PAGE4 and conformational switching: insights from molecular dynamics simulations and implications for prostate cancer. J Mol Biol. 2018;430:2422–2438. doi: 10.1016/j.jmb.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zeng Y, He Y, Yang F, Mooney SM, Getzenberg RH, Orban J, Kulkarni P. The cancer/testis antigen prostate-associated gene 4 (PAGE4) is a highly intrinsically disordered protein. J Biol Chem. 2011;286:13985–13994. doi: 10.1074/jbc.M110.210765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Diamandis EP, Yousef GM. Human tissue kallikreins: a family of new cancer biomarkers. Clin Chem. 2002;48:1198–1205. [PubMed] [Google Scholar]
- 92.Gallaher JA, Enriquez-Navas PM, Luddy KA, Gatenby RA, Anderson ARA. Spatial heterogeneity and evolutionary dynamics modulate time to recurrence in continuous and adaptive cancer therapies. Cancer Res. 2018;78:2127–2139. doi: 10.1158/0008-5472.CAN-17-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kulkarni P, Dunker AK, Weninger K, Orban J. Prostate-associated gene 4 (PAGE4), an intrinsically disordered cancer/testis antigen, is a novel therapeutic target for prostate cancer. Asian J Androl. 2016;18:695–703. doi: 10.4103/1008-682X.181818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Demokan S, Chuang AY, Pattani KM, Sidransky D, Koch W, Califano JA. Validation of nucleolar protein 4 as a novel methylated tumor suppressor gene in head and neck cancer. Oncol Rep. 2014;31:1014–1020. doi: 10.3892/or.2013.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takayanagi-Kiya S, Kiya T, Kunieda T, Kubo T. Mblk-1 transcription factor family: its roles in various animals and regulation by NOL4 splice variants in mammals. Int J Mol Sci. 2017;18:246. doi: 10.3390/ijms18020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takayanagi-Kiya S, Misawa-Hojo K, Kiya T, Kunieda T, Kubo T. Splicing variants of NOL4 differentially regulate the transcription activity of Mlr1 and Mlr2 in cultured cells. Zoolog Sci. 2014;31:735–740. doi: 10.2108/zs140049. [DOI] [PubMed] [Google Scholar]
- 97.Jiang C, Zhang Y, Li Y, Lu J, Huang Q, Xu R, Feng Y, Yan S. High CEP55 expression is associated with poor prognosis in non-small-cell lung cancer. Onco Targets Ther. 2018;11:4979–4990. doi: 10.2147/OTT.S165750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kalimutho M, Sinha D, Jeffery J, Nones K, Srihari S, Fernando WC, Duijf PH, Vennin C, Raninga P, Nanayakkara D, Mittal D, Saunus JM, Lakhani SR, Lopez JA, Spring KJ, Timpson P, Gabrielli B, Waddell N, Khanna KK. CEP55 is a determinant of cell fate during perturbed mitosis in breast cancer. EMBO Mol Med. 2018;10:e8566. doi: 10.15252/emmm.201708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gao XY, Wang XL. An adoptive T cell immunotherapy targeting cancer stem cells in a colon cancer model. J BUON. 2015;20:1456–1463. [PubMed] [Google Scholar]
- 100.Seyedabadi S, Saidijam M, Najafi R, Mousavi-Bahar SH, Jafari M, MohammadGanji S, Mahdavinezhad A. Assessment of CEP55, PLK1 and FOXM1 expression in patients with bladder cancer in comparison with healthy individuals. Cancer Invest. 2018;36:407–414. doi: 10.1080/07357907.2018.1514504. [DOI] [PubMed] [Google Scholar]
- 101.Hauptman N, Jevsinek Skok D, Spasovska E, Bostjancic E, Glavac D. Genes CEP55, FOXD3, FOXF2, GNAO1, GRIA4, and KCNA5 as potential diagnostic biomarkers in colorectal cancer. BMC Med Genomics. 2019;12:54. doi: 10.1186/s12920-019-0501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wolter P, Hanselmann S, Pattschull G, Schruf E, Gaubatz S. Central spindle proteins and mitotic kinesins are direct transcriptional targets of MuvB, B-MYB and FOXM1 in breast cancer cell lines and are potential targets for therapy. Oncotarget. 2017;8:11160–11172. doi: 10.18632/oncotarget.14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Waseem A, Ali M, Odell EW, Fortune F, Teh MT. Downstream targets of FOXM1: CEP55 and HELLS are cancer progression markers of head and neck squamous cell carcinoma. Oral Oncol. 2010;46:536–542. doi: 10.1016/j.oraloncology.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 104.Bastos RN, Barr FA. Plk1 negatively regulates Cep55 recruitment to the midbody to ensure orderly abscission. J Cell Biol. 2010;191:751–760. doi: 10.1083/jcb.201008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wei X, Chen F, Xin K, Wang Q, Yu L, Liu B, Liu Q. Cancer-testis antigen peptide vaccine for cancer immunotherapy: progress and prospects. Transl Oncol. 2019;12:733–738. doi: 10.1016/j.tranon.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krishnadas DK, Bai F, Lucas KG. Cancer testis antigen and immunotherapy. Immunotargets Ther. 2013;2:11–19. doi: 10.2147/ITT.S35570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang C, Gu Y, Zhang K, Xie K, Zhu M, Dai N, Jiang Y, Guo X, Liu M, Dai J, Wu L, Jin G, Ma H, Jiang T, Yin R, Xia Y, Liu L, Wang S, Shen B, Huo R, Wang Q, Xu L, Yang L, Huang X, Shen H, Sha J, Hu Z. Systematic identification of genes with a cancer-testis expression pattern in 19 cancer types. Nat Commun. 2016;7:10499. doi: 10.1038/ncomms10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yousef S, Heise J, Lajmi N, Bartels K, Kroger N, Luetkens T, Atanackovic D. Cancer-testis antigen SLLP1 represents a promising target for the immunotherapy of multiple myeloma. J Transl Med. 2015;13:197. doi: 10.1186/s12967-015-0562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zeng G, Li Y, El-Gamil M, Sidney J, Sette A, Wang RF, Rosenberg SA, Robbins PF. Generation of NY-ESO-1-specific CD4+ and CD8+ T cells by a single peptide with dual MHC class I and class II specificities: a new strategy for vaccine design. Cancer Res. 2002;62:3630–3635. [PMC free article] [PubMed] [Google Scholar]
- 110.Ostroumov D, Fekete-Drimusz N, Saborowski M, Kuhnel F, Woller N. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell Mol Life Sci. 2018;75:689–713. doi: 10.1007/s00018-017-2686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Al-Khadairi G, Decock J. Cancer Testis antigens and immunotherapy: where do we stand in the targeting of PRAME? Cancers (Basel) 2019;11:984. doi: 10.3390/cancers11070984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smith SM, Iwenofu OH. NY-ESO-1: a promising cancer testis antigen for sarcoma immunotherapy and diagnosis. Chin Clin Oncol. 2018;7:44. doi: 10.21037/cco.2018.08.11. [DOI] [PubMed] [Google Scholar]
- 113.Mackensen A, Herbst B, Chen JL, Kohler G, Noppen C, Herr W, Spagnoli GC, Cerundolo V, Lindemann A. Phase I study in melanoma patients of a vaccine with peptide-pulsed dendritic cells generated in vitro from CD34(+) hematopoietic progenitor cells. Int J Cancer. 2000;86:385–392. doi: 10.1002/(sici)1097-0215(20000501)86:3<385::aid-ijc13>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 114.Krishnadas DK, Bao L, Bai F, Chencheri SC, Lucas K. Decitabine facilitates immune recognition of sarcoma cells by upregulating CT antigens, MHC molecules, and ICAM-1. Tumour Biol. 2014;35:5753–5762. doi: 10.1007/s13277-014-1764-9. [DOI] [PubMed] [Google Scholar]
- 115.Esfandiary A, Ghafouri-Fard S. MAGE-A3: an immunogenic target used in clinical practice. Immunotherapy. 2015;7:683–704. doi: 10.2217/imt.15.29. [DOI] [PubMed] [Google Scholar]
- 116.Dhodapkar MV, Sznol M, Zhao B, Wang D, Carvajal RD, Keohan ML, Chuang E, Sanborn RE, Lutzky J, Powderly J, Kluger H, Tejwani S, Green J, Ramakrishna V, Crocker A, Vitale L, Yellin M, Davis T, Keler T. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci Transl Med. 2014;6:232ra251. doi: 10.1126/scitranslmed.3008068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mahipal A, Ejadi S, Gnjatic S, Kim-Schulze S, Lu H, Ter Meulen JH, Kenney R, Odunsi K. First-in-human phase 1 dose-escalating trial of G305 in patients with advanced solid tumors expressing NY-ESO-1. Cancer Immunol Immunother. 2019;68:1211–1222. doi: 10.1007/s00262-019-02331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baumgaertner P, Costa Nunes C, Cachot A, Maby-El Hajjami H, Cagnon L, Braun M, Derre L, Rivals JP, Rimoldi D, Gnjatic S, Abed Maillard S, Marcos Mondejar P, Protti MP, Romano E, Michielin O, Romero P, Speiser DE, Jandus C. Vaccination of stage III/IV melanoma patients with long NY-ESO-1 peptide and CpG-B elicits robust CD8(+) and CD4(+) T-cell responses with multiple specificities including a novel DR7-restricted epitope. Oncoimmunology. 2016;5:e1216290. doi: 10.1080/2162402X.2016.1216290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shi X, Chen X, Fang B, Ping Y, Qin G, Yue D, Li F, Yang S, Zhang Y. Decitabine enhances tumor recognition by T cells through upregulating the MAGE-A3 expression in esophageal carcinoma. Biomed Pharmacother. 2019;112:108632. doi: 10.1016/j.biopha.2019.108632. [DOI] [PubMed] [Google Scholar]
- 120.Konkankit VV, Kim W, Koya RC, Eskin A, Dam MA, Nelson S, Ribas A, Liau LM, Prins RM. Decitabine immunosensitizes human gliomas to NY-ESO-1 specific T lymphocyte targeting through the Fas/Fas ligand pathway. J Transl Med. 2011;9:192. doi: 10.1186/1479-5876-9-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oi S, Natsume A, Ito M, Kondo Y, Shimato S, Maeda Y, Saito K, Wakabayashi T. Synergistic induction of NY-ESO-1 antigen expression by a novel histone deacetylase inhibitor, valproic acid, with 5-aza-2’-deoxycytidine in glioma cells. J Neurooncol. 2009;92:15–22. doi: 10.1007/s11060-008-9732-0. [DOI] [PubMed] [Google Scholar]
- 122.Natsume A, Wakabayashi T, Tsujimura K, Shimato S, Ito M, Kuzushima K, Kondo Y, Sekido Y, Kawatsura H, Narita Y, Yoshida J. The DNA demethylating agent 5-aza-2’-deoxycytidine activates NY-ESO-1 antigenicity in orthotopic human glioma. Int J Cancer. 2008;122:2542–2553. doi: 10.1002/ijc.23407. [DOI] [PubMed] [Google Scholar]
- 123.Krishnadas DK, Wang Y, Sundaram K, Bai F, Lucas KG. Expansion of cancer germline antigen-specific cytotoxic T lymphocytes for immunotherapy. Tumour Biol. 2017;39:1010428317701309. doi: 10.1177/1010428317701309. [DOI] [PubMed] [Google Scholar]
- 124.Gjerstorff MF, Andersen MH, Ditzel HJ. Oncogenic cancer/testis antigens: prime candidates for immunotherapy. Oncotarget. 2015;6:15772–15787. doi: 10.18632/oncotarget.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]


