Figure 2.
Mechanisms Contributing to the Establishment of the Hyperactive State of the Nucleolus in ESCs
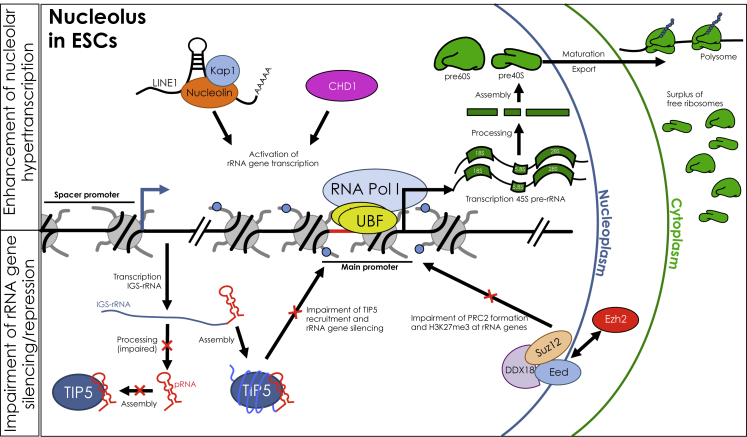
Hypertranscription of rRNA genes was shown to be favored by the binding of CHD1 (chromodomain helicase DNA-binding protein 1) (Guzman-Ayala et al., 2015) and LINE1-nucleolin RNA complex (Percharde et al., 2018) with rRNA genes. On the other hand, mechanisms for rRNA gene repression are impaired. The recruitment of TIP5 (TTF1-interacting protein 5) to rRNA genes is abrogated by the impairment of IGS-rRNA processing and consequent lack of formation of mature pRNA (promoter-associated RNA). The association of TIP5 with IGS-rRNA impairs TIP5 recruitment to rRNA genes and their epigenetic silencing (Leone et al., 2017; Savić et al., 2014). DDX18 (DEAD-box helicase 18) was shown to sequester PRC2 (polycomb repressive complex 2) in the outer layer of the nucleolus and impairs its formation (Zhang et al., 2020). This mechanism prevents the deposition of the repressive H3K27me3 mark onto rRNA genes. Hyperactivation of rRNA genes promotes ribosome biogenesis. However, ESCs require a low global protein synthesis rate. The enhanced ribosome biogenesis in ESCs might result in a surplus of free ribosomes, which can be used to allow rapid elevation of translation rate in response to differentiation signals (Golob et al., 2008; Sampath et al., 2008).