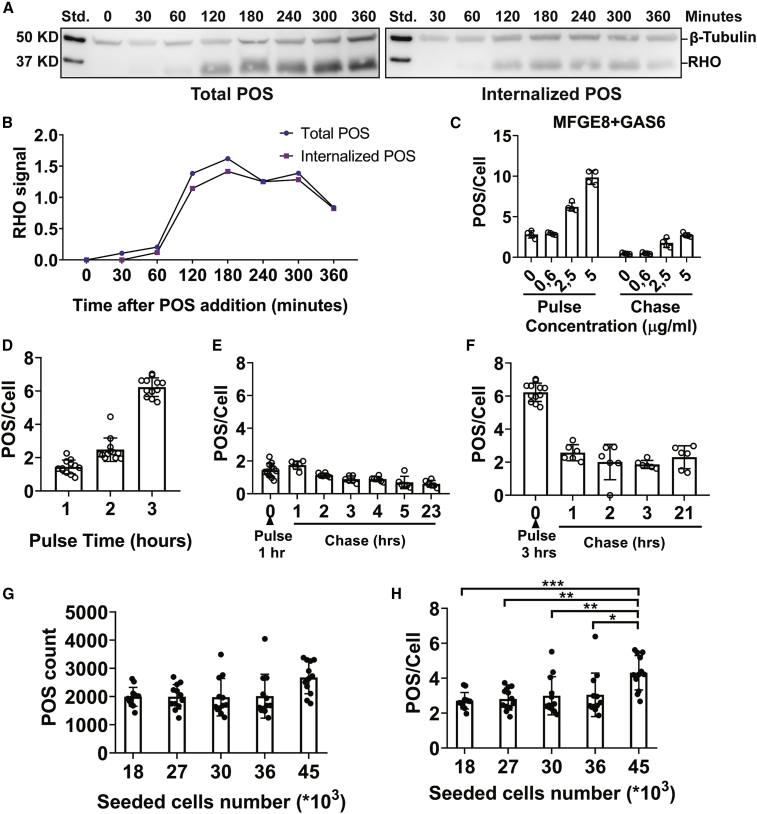
Figure 2.
Kinetics of Miniaturized POS Phagocytosis Assay Uptake and Degradation by Human RPE
(A) Western blot analysis of total and internalized POS by hESC-RPE over the course of 6 h. POS were added to RPE for 0, 30, 60, 120, 180, 240, 300, and 360 min and then cells were washed and treated with EDTA to detach bound POS and monitor internalized POS or with PBS to monitor total POS. Finally, cells were lysed and analyzed by western blot. Membranes were blotted with anti- RHO to monitor POS and β-tubulin as a loading control.
(B) Analysis of the western blot images in (A). Maximum binding and internalization is reached at 3 h following POS addition.
(C) POS were added to hESC-RPE in 384-well plates for 3 h in the presence of increasing concentrations of phagocytosis ligands, MFGE8+GAS6. At 3 h cells were either washed and fixed or left for 6 h more to monitor degradation of POS. Data represent the mean of the total POS/cell count ±SD. N = 4 RPE differentiation batches. RPE cells respond to ligand stimulation in a dose-dependent manner and they are able to degrade POS after 6 h.
(D) AF555-labeled POS were added to hESC-RPE for 1, 2, and 3 h and then cells were washed and fixed to monitor increase in total phagocytosed POS over time. Data represent the mean of the total POS/cell count ±SD. N = 4 RPE differentiation batches (three wells per batch).
(E and F) In the same experiment as in (D), separate wells were washed at 1 or 3 h and kept for 1, 2, 3, 4, 5, and 23 h or 1, 2, 3, and 21 h respectively to monitor POS degradation overtime.
(G) hESC-RPE cells were seeded on 384-well plates in increasing concentrations to obtain the optimal cell number for the phagocytosis assay. POS (106 POS/well) were seeded on the cells for 3 h before they were fixed and imaged. Data represent the mean of the total POS count ±SD. N = 3 RPE differentiation batches (three wells per batch). (H) Total POS count shown in (G) was divided over the nuclei count to obtain POS/cell count. Data represent the mean of the total POS/cell count ±SD. Significance was calculated using one-way-ANOVA comparing all samples with each other. Plating 45,000 cells/well increases the number of POS/cell significantly compared with the other concentrations.