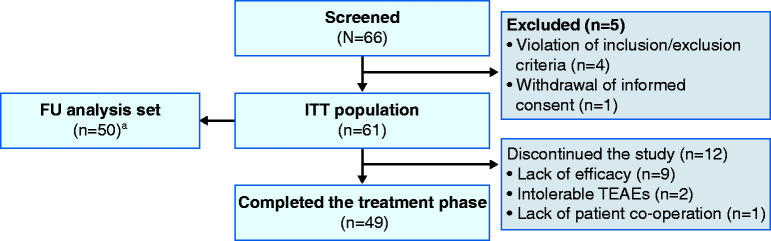
Figure 1.
Patient disposition.
aThe FU analysis set comprised all patients who received at least one dose of study drug during the FU phase and had at least one FU value for the safety endpoints to be analysed. Patients were not required to complete the treatment phase of the study to be included in the FU analysis set.
FU: follow-up; ITT: intention-to-treat; TEAE: treatment-emergent adverse event.