Figure 1. Flow of Participants in a Study of the Effect of Co-trimoxazole vs Placebo on Death, Lung Transplant, or Hospital Admission in Patients With Idiopathic Pulmonary Fibrosis.
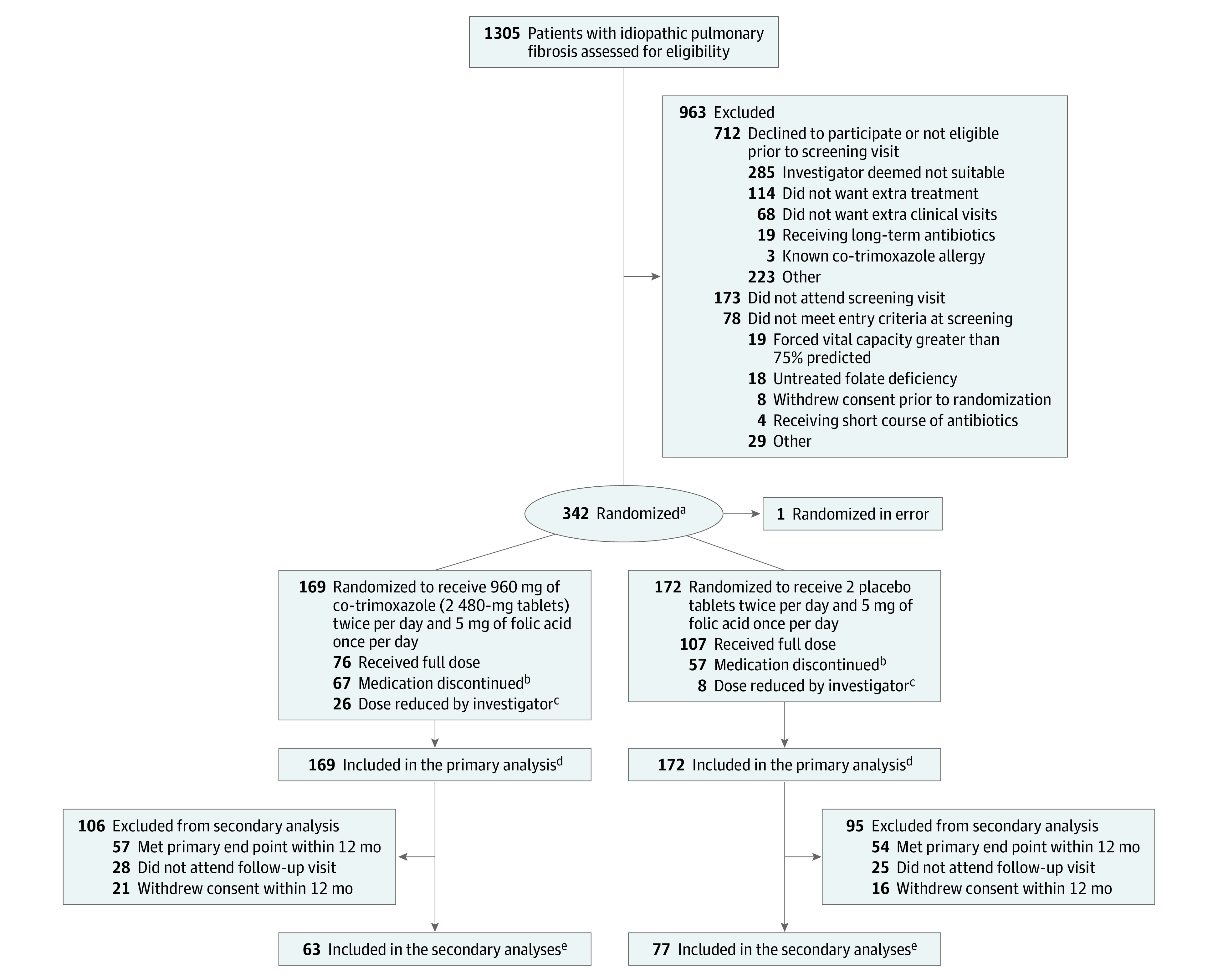
aIn a 1:1 ratio, with minimization for site and baseline fibrotic therapy.
bFive patients in the co-trimoxazole group and 8 in the control group previously had their dose reduced by the investigator.
cA reduction of the dose to 2 tablets (ie, 960 mg co-trimoxazole or 2 placebo tablets daily) plus 5 mg of folic acid 3 times weekly was permitted if a participant developed gastrointestinal adverse effects or rash, grade 1 hyperkalemia (potassium >5.0 mmol/L), or any other adverse event requiring dose reduction in the view of the principal investigator.
dA total of 32 individuals from the co-trimoxazole and 26 from the placebo group withdrew during the study, and their data are included until the point of withdrawal.
eThe secondary outcome data illustrate that of lung function.
