Fig. 5.
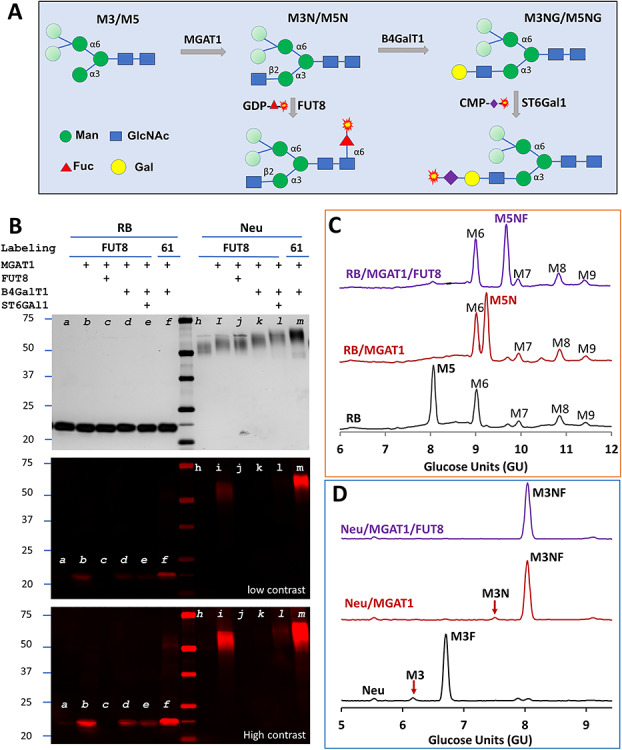
Detecting high-mannose glycans on glycoproteins by FUT8 and demonstration of the substrate specificity of FUT8. (A) Scheme for detecting high-mannose glycans. While the β1,2-linked GlcNAc introduced by MGAT1 on the α3 arm is essential for FUT8 recognition, the mannose residues in lighter shade on the α6 arm are flexible for substrate recognition. Further elongation of the α3 arm with B4GalT1 renders the glycan to be the substrate glycan for ST6Gal1. (B) Detecting Man5 on RNase B (RB) and Man3 on recombinant H1N1 neuraminidase monomer (Neu) and the substrate specificity of FUT8. Samples were pretreated with MGAT1, FUT8, B4GalT1 and ST6Gal1 with their respective unmodified donor substrates (indicated with +) before labeling. The pretreated samples were then labeled by FUT8 with GDP-Cy5-fucose or ST6Gal1 (61) with CMP-Cy5-Sialic acid. All samples were separated on SDS–PAGE and visualized by silver staining and fluorescent imaging. The middle and lower panels are the fluorescent image with different contrasts. Only MGAT1-modified sample was strongly labeled by FUT8, and further elongation reduced the labeling greatly. In parallel experiments, samples of RB (C) and Neu (D) were modified by MGAT1 or together with FUT8 with their natural donor substrates and then analyzed with GlyQ.
