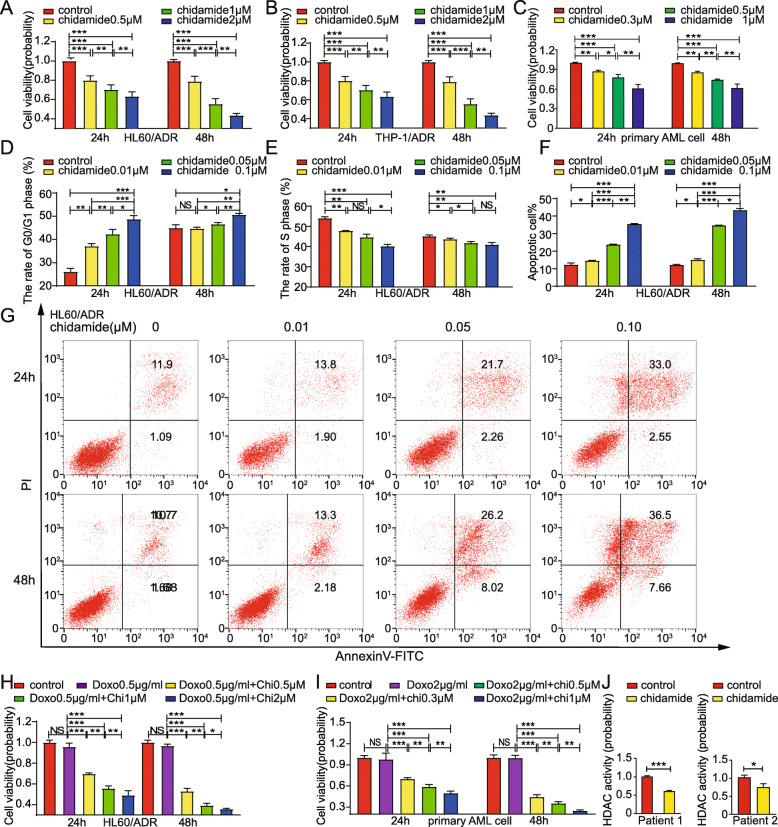
Fig. 4.
HL60/ADR, THP-1/ADR and patient-derived anthracycline-resistant AML cells are sensitive to either chidamide monotherapy or combination therapy of chidamide + doxorubicin. CCK-8 assays were used to assess the abilities of HL60/ADR (a), THP-1/ADR (b) and patient-derived cells (c) to proliferate. HL60/ADR G1 phase (d) and proportion of S phase (e) in response to incubation with chidamide were measured using flow cytometry. f-g are representatives of flow cytometry (Annexin V/PI) for detection of apoptosis. The cell viability of (h) HL60/ADR and (i) patients-derived primary AML cells treated by combination therapy were measured with CCK-8 assays. HDAC activity of anthracycline-resistant AML cells which were treated with chidamide (j). Data represents three independent experiments, results are shown in the format, mean ± S.D. (*P < 0.05, **P < 0.01, ***P < 0.001, NS: P > 0.05)