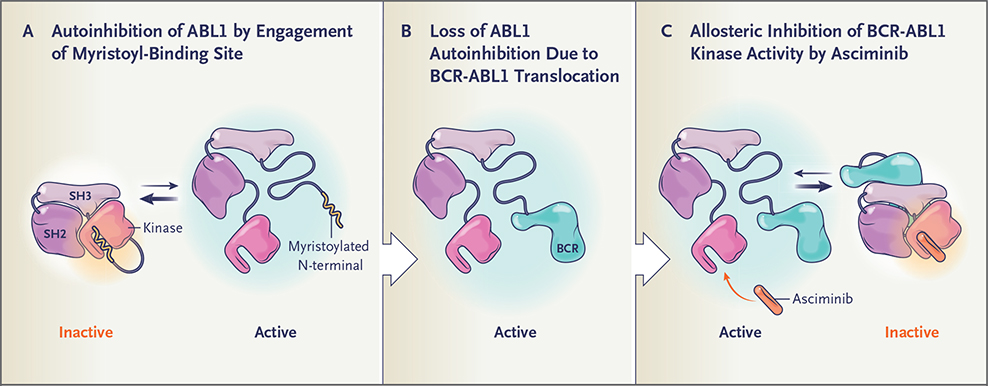
Figure 1. Binding of the Myristoyl Site of the BCR-ABL1 Protein by Asciminib.
Autoinhibition of the ABL1 kinase occurs through engagement of the myristoyl-binding site by the myristoylated N-terminal — a negative regulatory motif that locks the ABL1 kinase in the inactive state (Panel A). On fusion of ABL1 to BCR, the myristoylated N-terminal is lost and the ABL1 kinase is activated (Panel B). By allosterically binding the myristoyl site, asciminib mimics myristate and restores inhibition of BCR-ABL1 kinase activity (Panel C).