Figure 1.
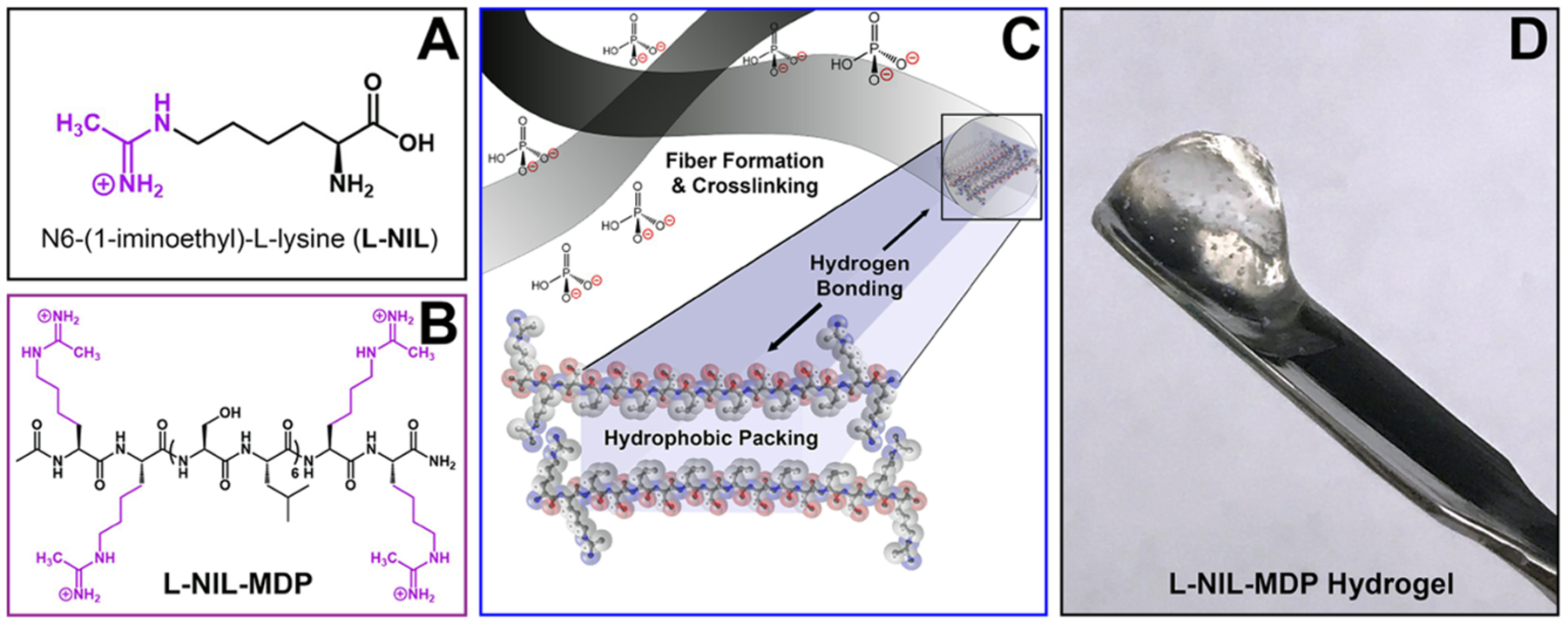
Chemical structures of the small-molecule drug N6-(1-iminoethyl)-l-lysine (l-NIL) (A) and the designed l-NIL multidomain peptide (B) chemically derived from the lysine-based peptide, K2(SL)6K2, by converting the starting material’s lysine side chains to l-NIL acetamidine functional groups. (C) Graphic depicting the nanofibers formed by self-assembly of multidomain peptides into antiparallel β-sheets, driven by hydrophobic packing and the formation of hydrogen-bonding networks between peptide backbones. Charged functional groups are displayed on the fiber surface and interact with phosphate counterions to extend and crosslink fibers. (D) Image of the easily manipulated hydrogel biomaterial that forms when the l-NIL-MDP is prepared at 1 wt % in phosphate-containing buffer.
