Figure 3.
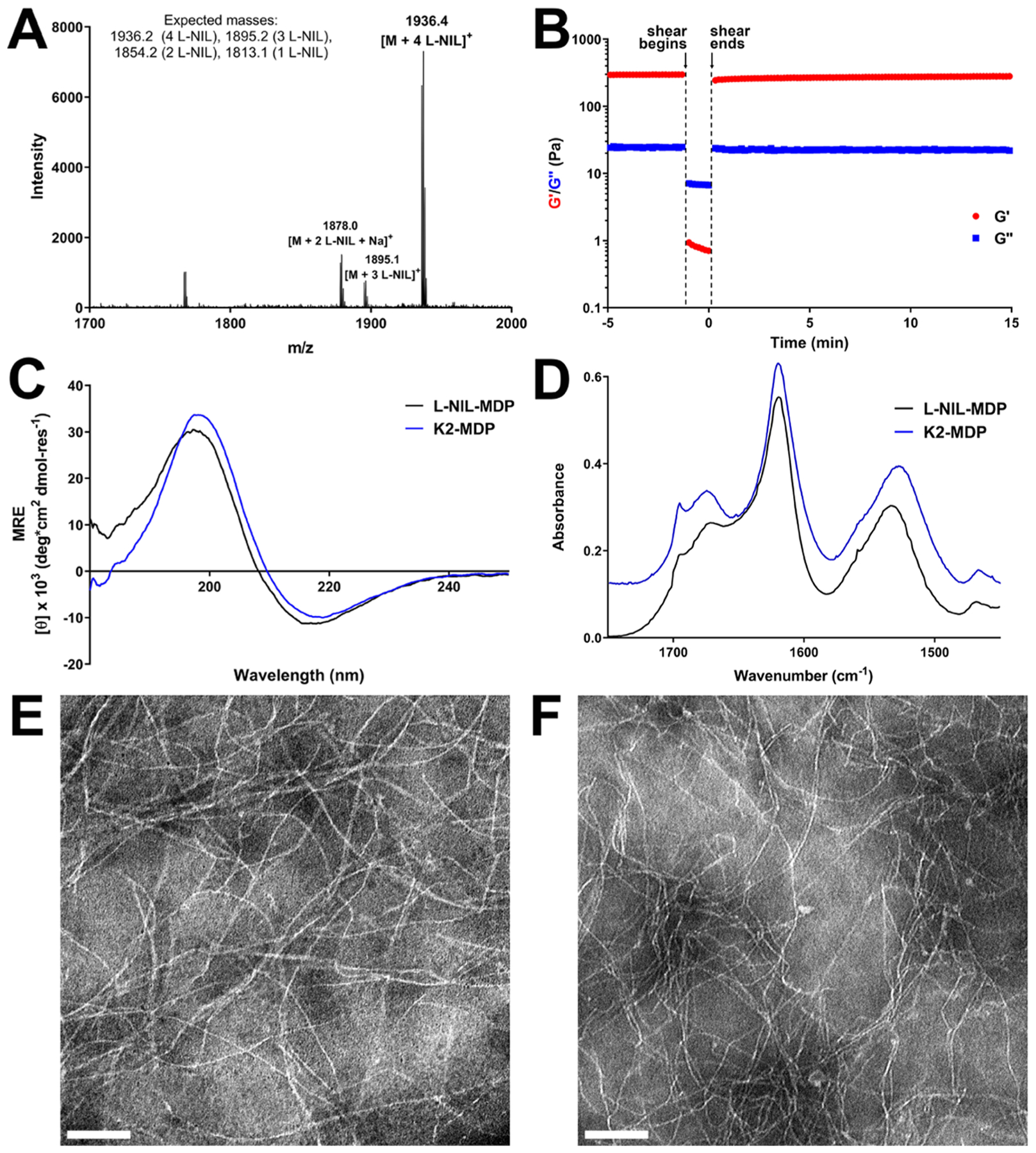
Chemical characterization data for the l-NIL-MDP biomaterial. (A) MALDI-mass spectrum showing the successful synthesis of l-NIL-MDP, primarily composed of a fully converted peptide (all four lysine side chains modified to l-NIL) with a small fraction possessing fewer modified side chains. (B) Oscillatory rheology showing the successful formation of l-NIL-MDP hydrogel with a storage modulus (G′) of ~300 Pa and loss modulus (G″) of ~25 Pa and with a shear recovery of ~86% 1 min after the shearing event. Circular dichroism (C) and attenuated total reflectance Fourier transform infrared spectroscopy (D) spectra (offset for clarity) confirmed the formation of the antiparallel β-sheet secondary structure that closely matches spectra for the parent peptide K2-MDP, demonstrating no significant change in the peptide structure upon conversion to l-NIL-MDP. Transmission electron microscopy images of (E) K2-MDP at 0.01 wt % and (F) l-NIL-MDP at 0.02 wt % shown at 40 000× magnification with scale bars = 100 nm.
