ABSTRACT
Situs inversus totalis (SIT) is a rare anomaly characterized by the transposition of organs. We present a case of a 67-year-old White woman with a history of SIT, who presented with fatigue, jaundice, and abnormal liver enzymes. Endoscopic ultrasound demonstrated a solid lesion at the distal common bile duct (CBD). Subsequent endoscopic retrograde cholangiopancreatography displayed severe stenosis in the CBD. A plastic stent was placed into the CBD, resulting in successful biliary decompression. Biliary brushings and biopsy showed atypical cells, suspicious for carcinoma. Ensuing pancreaticoduodenectomy confirmed cholangiocarcinoma. Although challenging, endoscopic ultrasound and endoscopic retrograde cholangiopancreatography in SIT can be successfully performed in preoperative evaluation for possible pancreaticobiliary cancers.
INTRODUCTION
Situs inversus totalis (SIT) is an autosomal recessive genetic condition, which is characterized by transposition of visceral organs across the midsagittal line from the more common position.1 The incidence of SIT is approximately 1 in 25,000 live births in the general population, with a male/female ratio of 3:2.2–4 Currently, it is acknowledged that SIT may be associated with other congenital anomalies.5 This difference in visceral location requires special attention when invasive interventions are planned. This is a case report of a patient with SIT who underwent endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasound (EUS) for the management of an indeterminate distal common bile duct (CBD) stricture.
CASE REPORT
A 67-year-old White woman was admitted with a 1-month history of obstructive jaundice. She also had a history of hyperlipidemia and hypertension. SIT in her was first discovered 5 years before presentation while undergoing an electrocardiogram. The electrocardiogram demonstrated a reversal of the expected lead polarity, and subsequent computed tomography (CT) scan confirmed the cardiologist's suspicion of SIT.
Six months before presentation for EUS and ERCP, the patient first began having vague complaints of fatigue and decreased appetite 6 months ago. Outpatient workup at that time showed no significant laboratory abnormalities (including liver enzymes), and an abdominal ultrasound was unremarkable. The patient's symptoms persisted, and 1 month before admission, she developed jaundice, elevated liver enzymes, and an abdominal CT scan showed a distended gallbladder, dilated intra/extrahepatic biliary tree, and a 1.5 cm obstructing lesion at the distal CBD without an associated mass in the pancreas or periportal area. On admission, laboratory tests showed normal complete blood count, total bilirubin 18.6 mg/dL (direct fraction of 15.4 mg/dL and indirect fraction of 3.2 mg/dL), alkaline phosphatase 570 U/L, alanine aminotransferase 143 U/L, aspartate aminotransferase 113 U/L, and CA 19-9 of 186 U/mL. On the CT scan, the pancreas was completely unremarkable with normal pancreatic duct without dilation or stricture. A 0.9 cm, a nonspecific lymph node was seen in the portocaval region without a definite metastatic disease.
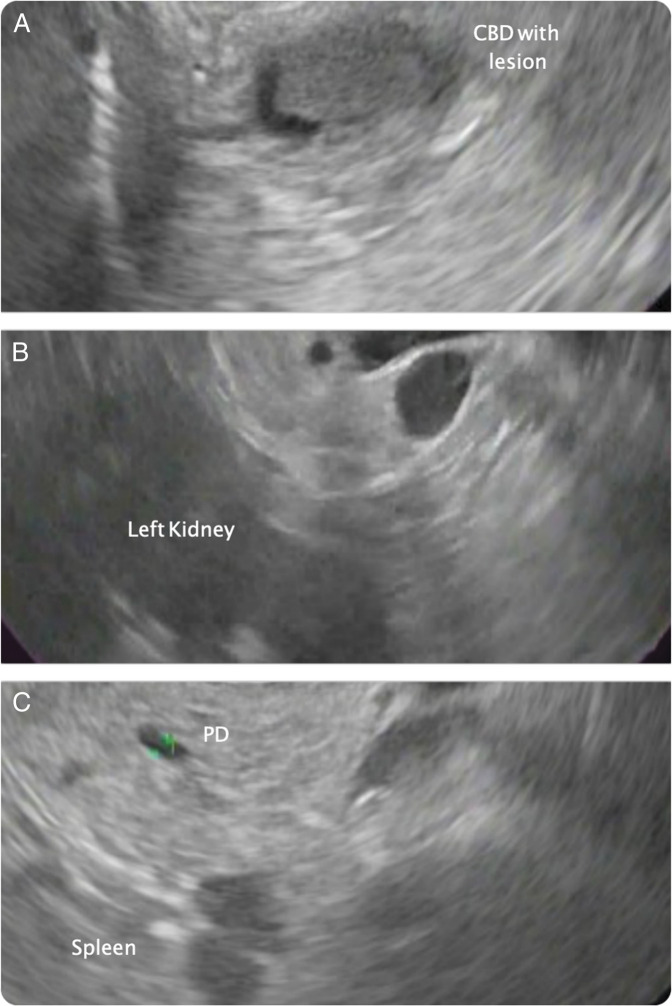
The patient's case was discussed a at multidisciplinary conference, and it was decided that the most prudent course of action would be to move forward with combined EUS and ERCP for diagnosis and relief of biliary obstruction. EUS was performed with the patient in a left lateral decubitus position and using a linear echoendoscope (Olympus GF-UCT180). All structures were identified as mirror images to their typical position and the echoendoscopy needed to be maneuvered with an understanding of the changes in anatomy. Within a few minutes, the scope maneuvering became somewhat natural, and all structures were identified. There was marked dilation of the common hepatic duct to 12 mm with a solid, hypoechoic, well-defined lesion with regular contours localized at the distal CBD measuring 11 mm in diameter. A reactive, hyperechoic, triangular lymph node was seen in the periportal region. No other abnormal findings were seen (Figure 1).
Figure 1.
Endoscopic linear ultrasound. (A) Duodenal window: presence of a solid, hypoechoic, well-defined formation at the distal common bile duct with inverse, counter-clockwise rotation of the scope in situs inversus totalis patient. (B) Gastric window: the pancreas and left kidney. (C) The spleen, pancreatic parenchyma with normal caliber pancreatic duct.
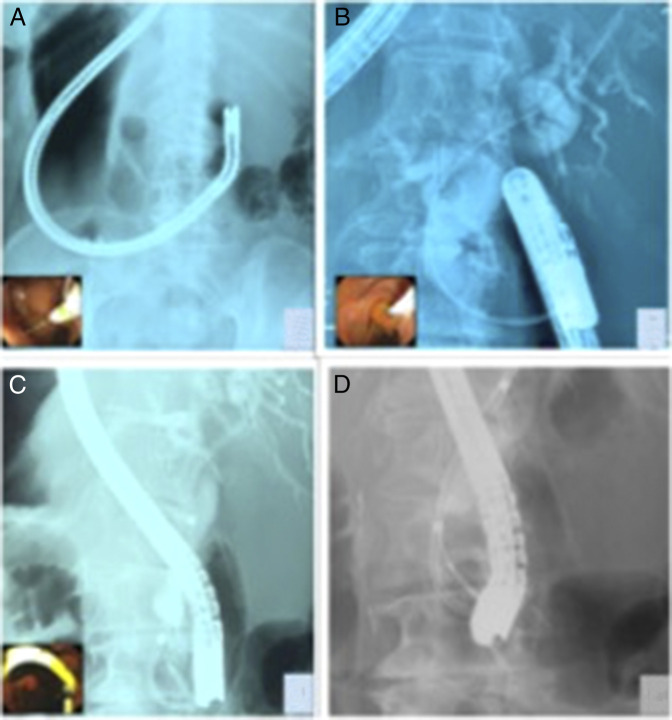
The ERCP was performed with the patient in the prone position and the endoscopist on the patient's right side using a side-viewing duodenoscope (Olympus TJF-Q180V). The duodenoscope was introduced through the mouth and advanced to the descending duodenum with moderate difficulty because of the inverted anatomy. The major papilla was identified by carefully reducing the scope counter clockwise. The bile duct cannulation was successful using a sphincterotome and a straight 0.035-inch guidewire in the usual pancreatic duct direction because of the mirror position of the organs. Injection of contrast after aspiration of the old bile revealed single severe stenosis at the distal CBD, which corresponded to the intraductal mass seen on EUS. A sphincterotomy was followed by the placement of a 10 Fr plastic stent, brushing, and biopsy. Adequate biliary decompression was evident by drainage of dark bile and remnant contrast media (Figure 2). The results from the histology and cytology showed atypical cells. The patient underwent a pancreaticoduodenectomy 7 days after her endoscopy. Her postoperative course was uncomplicated. The surgical specimen showed adenocarcinoma of the bile duct. Immunohistochemistry further characterized the pathology specimen as a high grade biliary intraepithelial neoplasia.
Figure 2.
Endoscopic cholangiopancreatography. (A) Fluoroscopy image demonstrated a mirror image to the expected duodenoscope position in a SIT patient. (B) Cholangiogram demonstrating proximal CBD dilation and distal stricture. (C) Double pig tail plastic stent placed in the CBD. (D) Tissue biopsy and brushing for histologic and cytology evaluation. CBD, common bile duct; SIT, situs inversus totalis.
DISCUSSION
SIT is a condition in which there is a reversal of the position of the abdominal and thoracic structures resulting in a mirror image of the commonly observed anatomy. There is no known association between SIT and gastrointestinal cancer. There are only 3 previously published case reports of cholangiocarcinoma with SIT.6–10
Organ et al. published the first case report in a 68-year-old woman with a distal CBD adenocarcinoma and SIT.6 ERCP showed a high-grade, 2-cm distal CBD stricture. The patient underwent pancreaticoduodenectomy followed by adjuvant chemotherapy and radiation. The patient died 18 months after surgery. Togliani et al reported a case of a 67-year-old man with cholangiocarcinoma and SIT.7 EUS was performed in the left lateral decubitus position. EUS showed a 2-cm hypoechoic mass in the distal CBD. EUS-fine needle aspiration showed cholangiocarcinoma, and subsequently, ERCP with biliary stenting was performed before pancreaticoduodenectomy.
Benhammane et al. reported a case of SIT and CBD adenocarcinoma in a 33-year-old man with obstructive jaundice.8 EUS showed a 15-mm hypoechoic mass at the distal CBD, and EUS-fine needle aspiration showed adenocarcinoma. After ERCP with biliary stenting, he underwent pancreaticoduodenectomy and adjuvant chemotherapy and radiation. He was alive at 8-month postsurgery (Table 1). In performing EUS and/or ERCP in SIT, some advocate patients being placed in the right lateral decubitus position to avoid potential technical difficulties.7 In our case, however, with the patient being in the left lateral decubitus position, all structures could be identified by EUS and ERCP performed without complications by carefully maneuvering the scopes as the anatomy allowed (Table 2). Our case illustrates that although SIT poses technical challenges in performing advanced endoscopic procedures, EUS and ERCP can be safely performed and are effective tools for tissue acquisition and biliary access.
Table 1.
Situs inversus with cholangiocarcinoma review of the literature
| Author | Age/sex | EUS-FNA, yes/no | ERCP/biliary stenting, yes/no | Surgery | Follow-up |
| Organ et al6 | 68/F | No | Yes | Pancreatico-duodenectomy | 18 mo |
| Benhammane et al8 | 33/M | Yes | Yes | Pancreatico-duodenectomy | 8 mo |
| Togliani et al7 | 67/M | Yes | Yes | Pancreatico-duodenectomy | N/A |
ERCP, endoscopic retrograde pancreatography; EUS, endoscopic ultrasound; FNA, fine needle aspiration.
Table 2.
Technical procedural differences in performing EUS and ERCP in a patient with situs inversus totalis
| Procedure steps | EUS with normal anatomy | ERCP with normal anatomy | EUS with SIT | ERCP with SIT |
| Position | Left lateral decubitus | Prone position | Left lateral decubitus | Prone position |
| Scope maneuver to enter the second portion of the duodenum | Move the tip of the scope to the right and upward | Move the tip of the scope to the right and up | Move the tip of the scope to the left and down | Move the tip of the scope to the left and down |
| Cannulation of the bile duct | N/A | The bile duct direction is usually at 11–12 o'clock position at the ampulla. | N/A | The bile duct direction is usually at 12–1 o'clock position at the ampulla. |
| Visualization of the bile duct | Pull the tip of the scope close to the wall at the duodenal bulb | If the contrast is injected into the ampulla, expect the cholangiogram to fill from the ampulla upward and slightly to the patient's right. | Pull the tip of the scope close to the wall at the duodenal bulb | If the contrast is injected into the ampulla, expect the cholangiogram to fill from the ampulla upward and slightly to the patient's left. |
| Visualization of the pancreas | Pull the scope slowly turning the scope clockwise | If the contrast is injected into the ampulla, expect the pancreatogram to fill from left to right. | Pull the scope turning the scope counterclockwise | If the contrast is injected into the ampulla, expect the pancreatogram to fill from right to left. |
ERCP, endoscopic retrograde pancreatography; EUS, endoscopic ultrasound; SIT, situs inversus totalis.
DISCLOSURES
Author contributions: M. Coronel and G. Lanke wrote the manuscript. D. Cambell, E. Coronel, and JH Lee revised the manuscript. C-WD Tzeng performed the surgical procedure. FW Chin reported the anatomopathological result. JH Lee is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
REFERENCES
- 1.Warwicker P, Goodship THJ, Donne RL, et al. Genetic studies into inherited and sporadic hemolytic uremic syndrome. Kidney Int 1998;53(4):836–44. [DOI] [PubMed] [Google Scholar]
- 2.Al-Jumaily M, Achab M, Hoche F. Laparoscopic cholecystectomy in situs inversus totalis: Is it safe? J Laparoendosc Adv Surg Tech A 2001;11(4):229–231. [DOI] [PubMed] [Google Scholar]
- 3.Lee SE, Kim HY, Jung SE, Lee SC, Park KW, Kim WK. Situs anomalies and gastrointestinal abnormalities. J Pediatr Surg 2006;41(7):1237–42. [DOI] [PubMed] [Google Scholar]
- 4.Peeters H, Devriendt K. Human laterality disorders. Eur J Med Genet 2006;49(5):349–62. [DOI] [PubMed] [Google Scholar]
- 5.Ali MS, Attash SM. Laparoscopic cholecystectomy in a patient with situs inversus totalis: Case report with review of literature. BMJ Case Rep 2013;2013:bcr2013201231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Organ BC, Skandalakis LJ, Gray SW, Skandalakis JE. Cancer of bile duct with situs inversus. Arch Surg 1991;126(9):1150–3. [DOI] [PubMed] [Google Scholar]
- 7.Togliani T, Pilati S, Mantovani N, et al. Extrahepatic cholangiocarcinoma in a patient with situs inversus totalis diagnosed by endoscopic ultrasound. Endoscopy 2013;45(Suppl 2 UCTN):E229–30. [DOI] [PubMed] [Google Scholar]
- 8.Benhammane H, Kharmoum S, Terraz S, et al. Common bile duct adenocarcinoma in a patient with situs inversus totalis: Report of a rare case. BMC Res Notes 2012;5:681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sano T, Kamiya J, Nagino M, Kanai M, Uesaka K, Nimura Y. Hepatectomy for proximal bile duct carcinoma in a patient with situs inversus; a case report. Hepatogastroenterology 2003;50(53):1266–8. [PubMed] [Google Scholar]
- 10.Tsunoda S, Miyashita T, Murata M. Pancreaticoduodenectomy for common bile duct cancer in a patient with situs inversus totalis: A case report. Int Surg 2006;91(1):24–7. [PubMed] [Google Scholar]