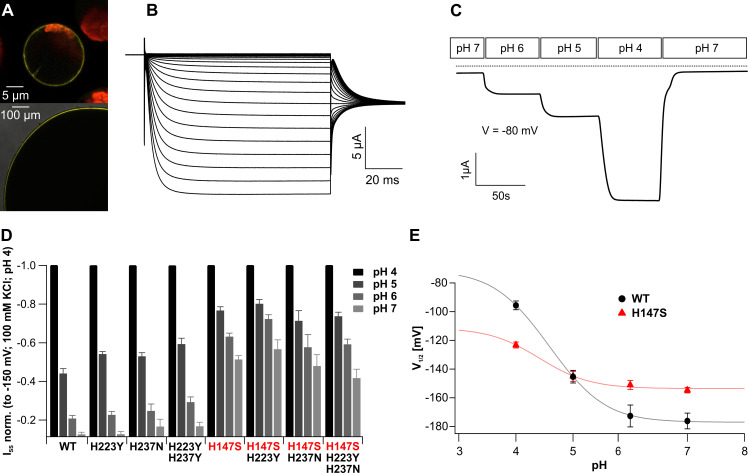
Fig 3. Functional properties of trigger hair–specific KDM1.
(A) Localization of YFP::KDM1 fusion constructs in the PM of A. thaliana mesophyll protoplasts (top) and X. laevis oocytes (bottom). A merged picture of YFP fluorescence and the chloroplast autofluorescence is presented in the upper panel. The lower panel shows a magnified image from an enlarged section of an oocyte to demonstrate the YFP signal on the surface of the membrane. Representative images are shown. (B) KDM1 cDNA confers functional expression of hyperpolarization-activated currents in X. laevis oocytes. Currents of up to −15 μA were elicited in response to hyperpolarizing pulses from a holding potential of 0 mV in 100 mM KCl bath solution. (C) Inward K+ currents elicited by a KDM1 expressing Xenopus oocyte markedly increased upon acidification of the external solution (Vm = −80 mV). The dotted line represents 0 current. (D) Comparison of the ISS at −150 mV of the indicated KDM1 channel mutants. ISS for each mutant were normalized to −150 mV at pH 4, which displays 100% activity. Note that mutants containing H147S are less affected by an external pH change at these voltages, whereas the other histidine mutants mediate WT-like K+ influx (n ≥ 6; mean ± SE). (E) The V1/2 of KDM1 WT and the H147S mutant was plotted against the applied pH values and fitted as described in the Materials and methods section with the following parameters: for mutant and WT, a = 0.6 and RT/F = 25.2 mV resulted in Vs = RT/(aF) = 42mV; for WT, V1/2_inf = −177.048 mV, pKO = 5.1328, pKC = 4.04; and for H147S, V1/2_inf = −153.632, pKO = 4.6285, pKC = 4.1914. The V1/2-pH curve of the mutant is compressed compared to that of the WT pointing to fundamental alterations in the pH-sensing process. (n ≥ 14; mean ± SE). The full raw dataset and the statistical analysis is provided in S3 Data. PM, plasma membrane; SE, standard error; WT, wild-type; YFP, yellow fluorescent protein.