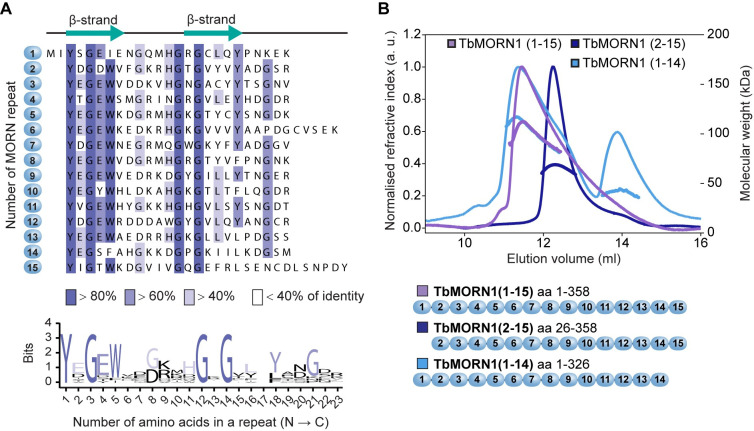
Fig 1. TbMORN1 primary structure and dimerisation.
(A) Primary structure of TbMORN1, with individual MORN repeats shown in alignment, and coloured according to amino acid conservation. A schematic of the predicted secondary structure of each repeat is shown above the alignment. A consensus amino acid sequence of the individual MORN repeats from TbMORN1 based on the alignment is shown in the sequence logo below. (B) TbMORN1 dimerises via its C-terminus. SEC-MALS profiles of TbMORN1(1–15), TbMORN1(2–15), and TbMORN1(1–14). Schematics are shown underneath. TbMORN1(1–15) tended to form high-order assemblies, whereas removal of the first MORN repeat resulted in a monodisperse dimer. Removal of the last MORN repeat in TbMORN1(1–14) resulted in a polydisperse mixture of monomers, dimers, and other species. Chromatographic separation was done using a Superdex 200 Increase 10/300 GL column, void volume 7.2 ml.