Abstract
BACKGROUND. With the advent of effective therapeutics, melanoma mortality rates have decreased, yet incidence rates are continuing to rise, making accurate prognostication for risk of recurrence increasingly important. Gene expression profiling (GEP) is a clinically available, objective metric that can be used in conjunction with traditional clinicopathological staging to help physicians stratify risk in melanoma patients. There is a gap in guidance from the American Joint Committee on Cancer (AJCC) and the National Comprehensive Cancer Network (NCCN) regarding how to utilize GEP in melanoma care. OBJECTIVE. An expert panel of 31-GEP test users sought to provide clarification of use options and a rational clinical workflow to guide appropriate application of the 31- GEP test in everyday practice. METHODS. The authors participated in an in-depth review of the literature and panel discussion regarding current limitations of melanoma risk assessment and opportunities for improvement with GEP. The panel reviewed 1) validation and clinical impact data supporting the use of sentinel lymph node biopsy (SLNB), 2) existing primary data and meta-analyses for 31-GEP testing in melanoma risk assessment, 3) AJCC, NCCN, and Melanoma Prevention Working Group (MPWG) data and guidelines for GEP use in melanoma risk assessment, and 4) experiences, rationales, and scenarios in which 31-GEP testing may be helpful for risk assessment. RESULTS. The 31-GEP test is useful and actionable for patient care when applied in accordance with current NCCN guidelines. Stratification of patients into low (Class 1a), intermediate (Class 1b or 2a), or high (Class 2b) risk categories can inform multidisciplinary conference discussion and can assist with determining the intensity of imaging, surveillance, and follow-up care. Patient-specific features of the disease and individual circumstances should be considered in the decision to use 31-GEP testing. CONCLUSION. The authors suggest a clinical workflow that integrates 31-GEP testing under the umbrella of current national guidelines. Application of the test in appropriate patient populations can improve risk assessment and inform clinical decision-making.
Keywords: Melanoma, gene expression profiling, 31-GEP, risk stratification, prognosis, prognostic algorithm, NCCN
With the emergence of immune checkpoint inhibitor therapy, patients with melanoma are experiencing greater survival rates, and there is an important prognostic need to identify patients with advancing disease.1 The American Joint Committee on Cancer (AJCC) staging criteria2 are designed to provide clinically relevant prognostic information based on features of the tumor (e.g., depth, ulceration) and metastasis to lymph nodes or other organs. These criteria are based on data from over 46,000 patients with Stage I, II, or III melanoma.2 AJCC staging is clinically helpful in determining appropriate surgical margins, sentinel lymph node biopsy (SLNB) eligibility, and whether systemic therapy should be offered to patients with melanoma. However, there is room for improvement.
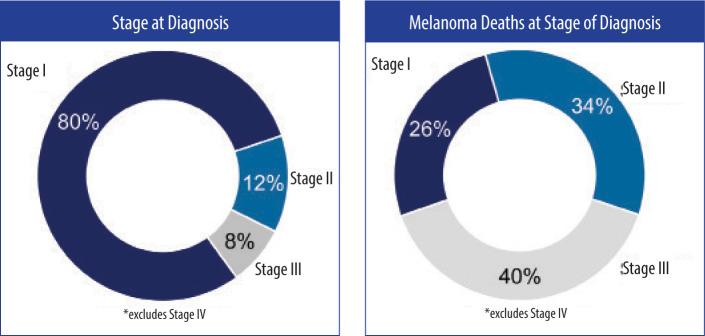
For example, the majority of melanoma-related deaths occur in early stage (localized Stage I–II at time of diagnosis) disease (Figure 1).3 AJCC staging2 and National Comprehensive Cancer Network (NCCN) guidelines4 are not able to identify or better triage the lethal melanomas that fall into the early stage categories. Additionally, the prognosis for patients with Stage IIIA (85.6% 10-year survival) disease is better than that for those with Stage IIB (83.3% 10-year survival) and Stage IIC (75.1% 5-year survival).3 Together, these data reveal an important gap in our ability to refer SLNB-negative high-risk patients for potential life-saving adjuvant therapy or save lower-risk SLNB-positive populations from substantial adjuvant therapy-induced adverse effects.
FIGURE 1.
While American Joint Commission on Cancer (AJCC) clinicopathologic factors are helpful clinically, the majority of deaths occur in early stage disease in patients with cutaneous melanoma3
© 2020 Castle Biosciences
In an effort to establish some rational guidance to clinicians on the use of gene expression profiling (GEP) testing, in the absence of specific melanoma-related GEP recommendations, an expert panel comprising academic and community dermatologists with experience using the 31-GEP test and familiarity with published studies in this area, as well as a medical representative from Castle Biosciences, convened on May 23, 2020. The focus of the group was on the 31-GEP test (DecisionDx-Melanoma, Castle Biosciences, Friendswood, Texas), which is commercially available in the United States (US) and covered by Medicare. Current literature reports validation5–7 and prospective data,8–10 as well as data on the clinical impact of GEP in melanoma management.11–14 Meta-analyses have likewise been published supporting the use of 31-GEP in melanoma risk stratification.15–19 This article presents the panel’s discussions on 31-GEP, including a review of pertinent GEP data, and attempts to bridge a gap for the practicing dermatologist by suggesting ways to implement 31-GEP testing in different clinical situations within current AJCC staging and integrate it into the NCCN guidelines for cutaneous melanoma.2,4
PROGNOSTIC LIMITATIONS OF SLNB
The overlap between Stage II and Stage III survival rates can be explained by the limitations of the predictive tools currently being used for melanoma. The current gold standard in melanoma prognostication is the SLNB, an independent prognostic biomarker for patients with cutaneous melanoma. The NCCN Clinical Practice Guidelines in Oncology: Cutaneous Melanoma (v 3.2020)4 provides guidance on patient selection for SLNB testing (Table 1). The landmark MSLT-1 study clearly demonstrates the prognostic value SLNB testing offers patients with melanoma.20,21 Despite the clear value of SLNB, this study also reported 83 deaths in the 643-participant node-negative group and 41 deaths in the 122-participant node-positive group. This shows that, overall, over twice as many patients who developed metastatic disease and later died had a negative SLNB (in part because the number of node-negative participants was so much higher in the study).20,21
TABLE 1.
Summary of NCCN recommendations for sentinel lymph node biopsy (SLNB) in patients with cutaneous melanoma7
|
|
|
The NCCN guidelines recognize the importance of a positive SLN as a negative prognostic factor and emphasize high-frequency clinical management that includes increased imaging, clinical surveillance, and consideration for adjuvant therapy; however, there is uncertainty regarding the management of patients with a negative SLN. This is reflected in nonspecific guidance that indicates wide clinical intervals for clinical surveillance and imaging. With the rapid improvement in systemic therapies for melanoma, early detection of metastatic disease is imperative; thus, there is urgent need for additional objective factors that can prognosticate the risk of recurrence—and be used within the broad guidance from the NCCN—to help guide clinical surveillance, imaging, and management of patients with cutaneous melanoma.
GENE EXPRESSION PROFILING: DATA SUMMARY
The 31-GEP test is an independent prognostic tool that is based on a gene transcription assay, specifically the genomic analyses of cutaneous malignant melanoma tumors and selected genes with particularly significant expression in metastatic cutaneous melanoma.6 The test evaluates 28 signature and three control genes using reverse transcription polymerase chain reaction (RTPCR) on primary tumor tissue from a confirmed diagnosis of Stage I, II, or III melanoma. An algorithm6 then accurately classifies patients as Class 1a (low risk), Class 1b/2a (moderate risk), or Class 2b (high risk) (*note that Class 1b/2a are combined into an intermediate grouping).
GEP testing has the potential to help close the current prognostic gap. As an objective metric, it might be used to help healthcare providers further risk-stratify patients with melanoma based on their individual tumor biology. The authors reviewed the existing 31-GEP test literature to assess potential benefits of adding GEP to the prognostic melanoma armamentarium, particularly with respect to high-risk SLNB-negative patients who are at high risk for metastasis due to their tumor biology rather than node status.22–24
Evidence supporting use of 31-GEP. Pertinent data from clinical studies are reviewed and discussed below. See Table 2 for complete list of studies with summarized data.5,6,8,9,15,22,26–29
TABLE 2.
| STUDY | METHODS | KEY FINDINGS |
|---|---|---|
| Clinical validity and performance—risk of recurrence | ||
| Gerami et al6 (January 2015) | Multicenter (6 sites), archival, N=268, Stage I–IV melanoma |
|
| Gerami et al25 (May 2015) | Multicenter (7 sites), archival, N=217, Stage I–IV melanoma (all underwent SLNB) |
|
| Zager et al5 (February 2018) | Multicenter (16 sites), archival, N=523 |
|
| Gastman et al22 (January 2019) | Multicenter (18 sites), archival, N=690; combined non-overlapping cohort of patients from Gerami6, Gerami,25 and Zager5 that had 31-GEP results, staging information, and survival outcomes; Stage I–III melanoma |
Combined cohort (n=690)
|
| Greenhaw et al15 (March 2020) | Meta-analysis of retrospective and prospective studies, N=1,479, Stage I–III |
|
| Where to use the test—risk of recurrence | ||
| Marks et al26 (July 2019) | Multicenter, archival, prospective, N=1,479, based on combined cohorts Gastman8, Greenhaw,15 Hsueh,37 and new archival cohort |
|
| Clinical validity and performance—SLNB guidance | ||
| Vetto et al27 (January 2019) | Multicenter (26 sites), prospective, n=1,421; limited to T1–T2 tumors, n= 1,065) |
|
| Prospective studies—risk of recurrence | ||
| Hsueh et al28 (January 2017) | Multicenter (11 sites), prospective, N=322; combination of INTEGRATE and EXPAND studies that include both dermatological and surgical centers |
|
| Greenhaw et al.8 (December 2018) | Single center, retrospective/prospective, N=256, dermatologic practice |
|
| Podlipnik et al29 (February 2019) | Multicenter (5 sites), prospective, N=86, dermatologic practices in Spain |
|
| Keller et al9 (April 2019) | Single center, prospective, N=159, surgical oncology practice |
|
*DecisionDx-Melanoma® 31-GEP test (Castle Biosciences, Friendswood, Texas)
**2,678 unique patients from the studies listed in the table, with over 5,700 patients in entire body of evidence
AJCC: American Joint Committee on Cancer; DFS: disease-free survival; DM: distant metastasis; DMFS: distant metastasis-free survival; GEP: gene expression profile; KM: Kaplin-Meier; MSS: melanoma-specific survival; NPV: negative predictive value; N: number; OS: overall survival; RFS: recurrence-free survival; SLN: sentinel lymph node; SLNB: sentinel lymph node biopsy
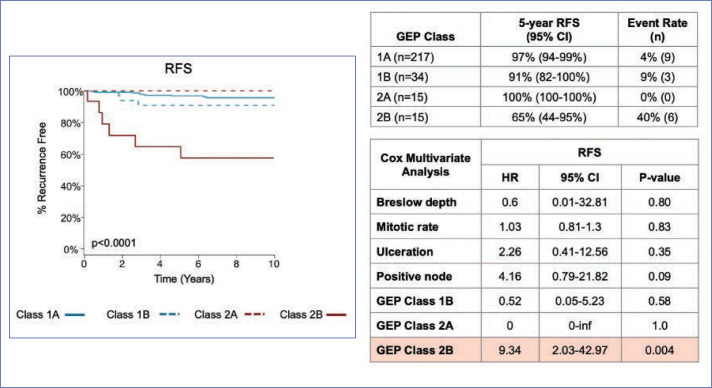
Validation data. In a study by Gastman et al,22 investigators examined 31-GEP test results from a pooled validation cohort of 690 patients with melanoma in an effort to identify those within subgroups who were considered low risk by standard criteria, including negative SLNB result, thin tumor (<1mm [T1]), and Stage I–IIA disease (NCCN low risk group) (Figure 2). The investigators reported that, based on Kaplan-Meier and Cox regression analyses, 70 percent of the patients in the cohort who had negative SLNB results experienced metastasis as Class 2 (70% sensitivity), and concluded that the 31-GEP is an independent predictor of risk compared to standard staging criteria in Stage I–IIA patients. One paper has criticized the use of 31-GEP in <1mm melanoma populations.19 Therefore, the expert panel specifically reviewed the value of the GEP in this subgroup related to the Gastman et al22 and Marks et al26 data. The Gastman paper had 281 melanomas <1mm in depth with 15 Class 2b test results. Here, the authors found the Class 2b result to be the only factor to reach statistical significance in a multivariate analysis, with a hazard ratio of 9.3 for recurrence-free survival (RFS), while the accompanying Kaplan-Meier curve showed a clear significant separation of risk between Class 2b patients and the rest of the 31-GEP results (Figures 2–4).22 Although the number of <1mm melanoma cases is relatively small, the same rate of 2b outcomes was replicated in the Marks et al26 cumulative modeling study. In both studies, the 31-GEP test provided additional information about aggressive biological behavior that can be superimposed upon thin (<1mm) melanomas.
FIGURE 2.
31-GEP identifies patients at high risk of melanoma recurrence and distant metastasis in patients with thin (≤1mm ) tumors
GEP: gene expression profiling; RFS: recurrence-free survival
Graphs reproduced with permission. Gastman BR, Gerami P, Kurley SJ, Cook RW, Leachman S, Vetto JT. Identification of patients at risk of metastasis using a prognostic 31-gene expression profile in subpopulations of melanoma patients with favorable outcomes by standard criteria. J Am Acad Dermatol. 2019;80(1):149-157.
FIGURE 4.
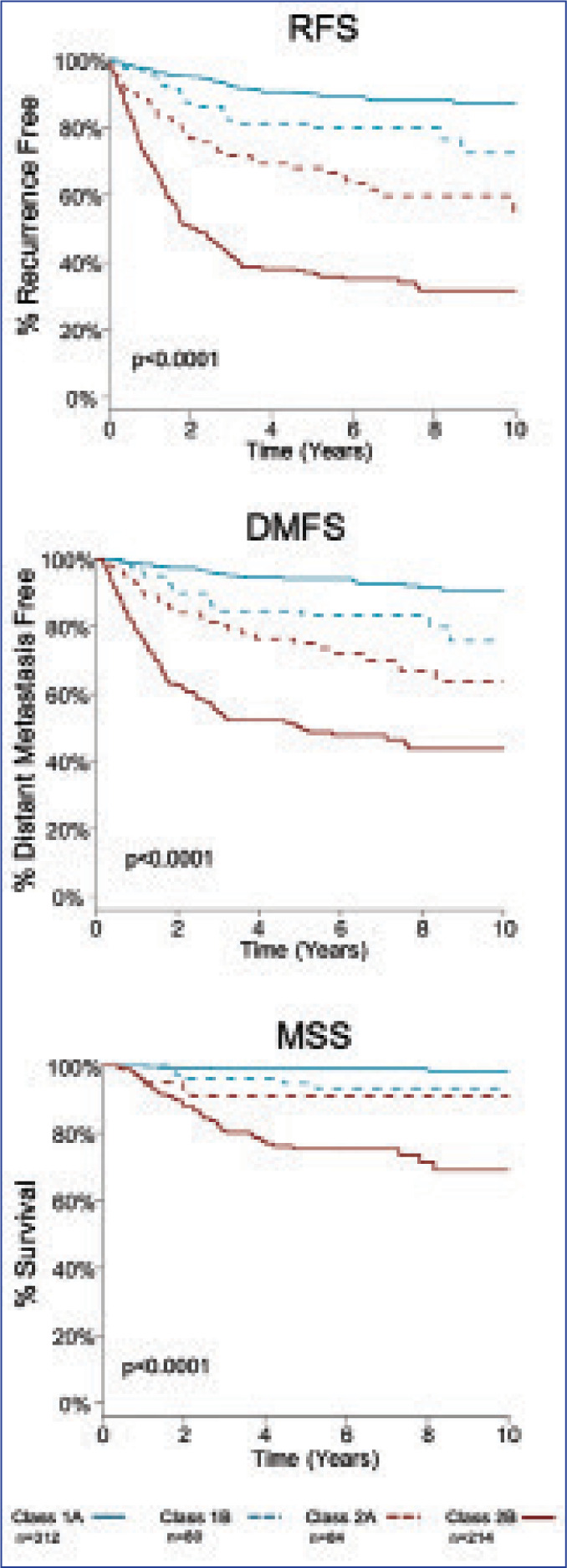
Clinical validity and prognostic value of 31-GEP in 690 patients with Stages I–III melanoma— median time to distant recurrence for patients with Class 2 result: Stage I–II patients, 1.6 years (70 Class 2 patients recurred); Stage III patients, 1.0 year (92 Class 2 patients recurred)
RFS: recurrence-free survival; DMFS: distant metastasis-free survival; MSS: melanoma-specific survival
Graphs reproduced with permission. Gastman BR, Gerami P, Kurley SJ, Cook RW, et al. Identification of patients at risk of metastasis using a prognostic 31-gene expression profile in subpopulations of melanoma patients with favorable outcomes by standard criteria. J Am Acad Dermatol. 2019;80(1):149–157.
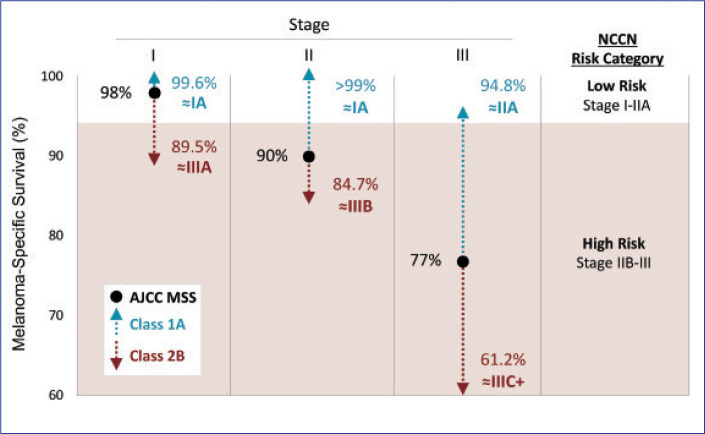
FIGURE 3.
Data from cohort study (N=690) demonstrated that the 31-GEP test further informs risk analysis obtained by the American Joint Committee on Cancer (AJCC) staging protocol in patients with melanoma
MSS: melanoma-specific survival; NCCN: National Comprehensive Cancer Network
Reproduced with permission. Gastman BR, Gerami P, Kurley SJ, et al. Identification of patients at risk of metastasis using a prognostic 31-gene expression profile in subpopulations of melanoma patients with favorable outcomes by standard criteria. J Am Acad Dermatol. 2019;80(1):149–157.
Prospective data. In an independent study assessing the predictive accuracy of the 31-GEP test, Greenhaw et al8 sought to determine the clinical and histopathologic features that predict high-risk classification in patients with melanoma. The investigators also evaluated how intermediate classes (1b and 2a) of patients with melanoma performed clinically. Greenhaw et al analyzed clinical, histopathologic, and outcomes data from a single-center melanoma patient registry that was collected prospectively by their institution. Specifically, data from patients with melanoma who had been treated within the last five years and undergone GEP testing were included in the analysis. A subcohort of patients with known metastatic disease was also identified and tested. The data analysis indicated that the 31-GEP test accurately identified 77 percent of the metastatic cutaneous melanomas as high risk (Class 2), with a negative predictive value of 99 percent for Class 1 cutaneous melanomas. Additionally, the analysis showed that cutaneous melanomas rated as Class 2 by the 31-GEP test were 22 times more likely to metastasize (Table 3). The investigators reported that the results of this independent, prospective data analysis were in agreement with previously reported data from industry-sponsored studies; they concluded that 31-GEP testing, when used in conjunction with current AJCC staging embedded within the NCCN melanoma guidelines, is a useful prognostic tool that can help direct optimal care provided by clinicians for their patients with melanoma.8
TABLE 3.
Independent and combined accuracy metrics of GEP and SLNB in patients with GEP results and SLNB status
| METRICS | GEP % (95% CI) | SLNB % (95% CI) | GEP & SLNB % (95% CI) |
|---|---|---|---|
| RFS | n=1,479 | n=867 | n=867 |
| Sensitivity | 76 (71–80) | 57 (51–63) | 88 (84–92) |
| Specificity | 76 (73–78) | 74 (70–77) | 52 (48–56) |
| DMFS | n=1,223 | n=867 | n=867 |
| Sensitivity | 76 (70–82) | 61 (55–68) | 90 (85–94) |
| Specificity | 69 (66–72) | 72 (68–75) | 48 (44–52) |
CI: Confidence interval; DMFS: distant metastasis-free survival; GEP: gene expression profile; RFS: recurrence-free survival; SLNB: sentinel lymph node biopsy
Adapted from Greenhaw BN, Covington KR, Kurley SJ, et al. Molecular risk prediction in cutaneous melanoma: a meta-analysis of the 31-gene expression profile prognostic test in 1,479 patients. J Am Acad Dermatol.202083(3):745-753.
Clinical impact data. According to four clinical impact studies,11,13,25,30 the addition of 31-GEP to current melanoma staging protocol consistently impacts clinical management decisions for one out of every two patients11 with melanoma who received the test. Management changes include frequency of clinical visits, use of imaging and lab tests, referrals to specialists, and use of SLNB. Physicians use the test results of 31-GEP, together with AJCC staging within NCCN guidelines, “to guide risk-appropriate treatment decisions that match [each individual patient’s] biological risk of the tumor.”11
In the multicenter, clinical impact study by Dillon et al,11 researchers sought to evaluate the impact of 31-GEP testing on clinical management of patients with melanoma. Data, including clinical features of the primary melanoma and recommendations for clinical follow-up and surveillance, were collected from physicians at 16 dermatology, surgical, or medical oncology centers. Recommendations for clinical management were again collected following administration of the 31-GEP test. Pre-test recommendations were compared to post-test recommendations to determine if the additional information provided by GEP testing, when considered in conjunction with AJCC staging, resulted in clinical management changes. The investigators reported that, overall, changes in treatment plans occurred in 49 percent (122 out of 247 patients) of the cases included in the study, compared to pre-test plans, with changes occurring in 36 percent of Class 1 cases and 85 percent of Class 2 cases. The results of the study indicated that GEP class was a significant factor for changes in a patient’s care plan ( p<0.001)—A Class 1 result accounted for 91 percent of the cases decreasing in management intensity, while Class 2 accounted for 72 percent of cases increasing in management intensity.11
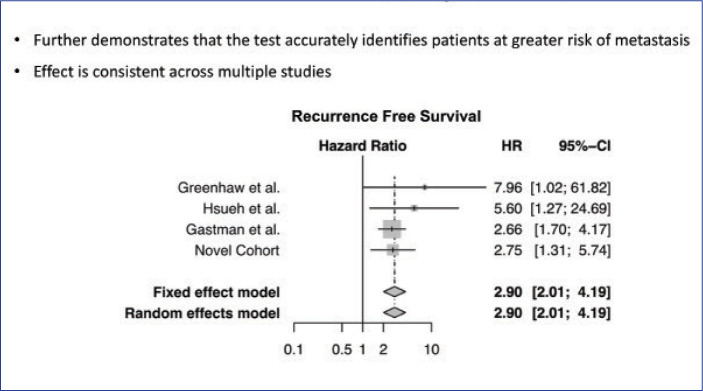
Level of evidence. In a meta-analysis, Greenhaw et al15 sought to determine the overall effect of 31-GEP, as well as its clinical outcome data, compared to AJCC staging, in patients with melanoma. Data from three studies and a novel cohort were included in the analysis (n=1,479). Investigators reported five-year RFS and distant metastasis-free survival (DMFS) rates of 91.4 percent and 94.1 percent for Class 1a patients, respectively, and 43.6 percent and 55.5 percent for Class 2b patients, respectively (p<0.0001). Class 2 was significantly associated with recurrence (p<0.0001) and distant metastasis (p<0.0001). The investigators also reported that 31-GEP identified AJCC Stage I–III patient subsets at high risk for recurrence and distant metastasis, with a sensitivity of 76 percent (95% confidence interval [CI] 71–80%) and 76 percent (95% CI 70–82%) for each endpoint, respectively. When 31-GEP and SLNB results were considered together, DMFS sensitivity and negative predictive values were both improved (Figure 5).15
FIGURE 5.
Results of 31-GEP meta-analysis of 4 patient cohorts demonstrated that the test accurately identified patients at greater risk of metastasis and that the effect was consistent across multiple studies.
A recent meta-analysis by Marchetti et al,19 which discussed level of evidence for GEP tests, found the 31-GEP to have stage-specific prognostic value with less potential for contributing in a major way to earlier stage disease. This study had several limitations. The study only compared Class 1 to Class 2 patients and did not differentiate patients who clearly had the worst prognosis (Class 2b). As seen in Figure 4, Class 2b has the worst associated prognosis and is an independent risk factor of melanoma RFS, DMFS, melanoma-specific survival (MSS). Additionally, the study excluded subsets of patients with melanoma (i.e., Stage III patients were not included in the analysis).19 We are in agreement with the authors that it is important to review and establish rationale via additional studies for which early stage patients will most benefit from 31-GEP.
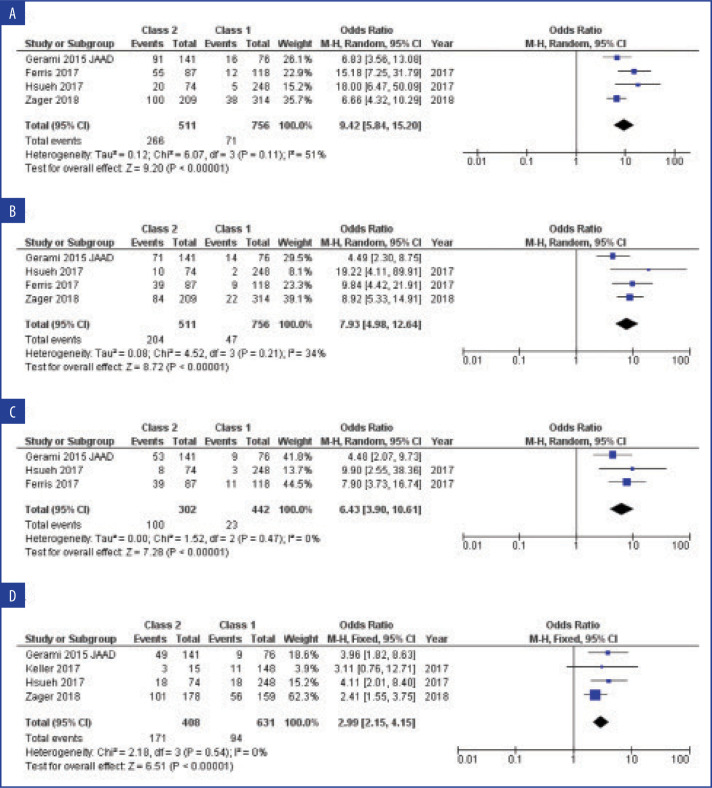
In a systematic review and meta-analysis by Litchman et al,18 investigators evaluated studies on GEP in primary melanoma prognosis and assessed GEP signatures for prognostic and analytic validity and clinical impact. The authors found 29 articles consisting of nine gene signatures and conducted a meta-analysis on six studies on a 31-gene signature. The results of their analysis indicated that high-risk GEP status was associated with poorer RFS, DMFS, and overall survival and SLNB positivity (Appendix 1).18
Overall 31-GEP data summary. For the authors of the current article, the most compelling aspect of the data was not found in a single study or meta-analysis. The consistency of the results across virtually every study, whether performed at a single site, multiple centers, sponsored or independent, was reminiscent of early data used to support use of the SLNB prognostic test prior to publication of prospective trials. Similarly, the 31-GEP test can help provide additional data to guide decision making in a variety of clinical scenarios for patients with melanoma. Dermatologists routinely oversee the long-term management of localized melanoma, and, in consultation with their melanoma care team, need guidance to aid in medical decision making tailored to the needs of their individual patients, particularly those with Stage I–III cutaneous melanoma. Taken together, the expert panel agreed that, ideally, additional prospective studies should be performed, but the current data, test availability, and ongoing use of GEP testing in practices across the US makes guidance regarding its use imperative. In summary, GEP testing may be used as a surrogate marker for aggressive biological behavior given its clear differentiation by class, with the worst prognosis being clearly associated with a Class 2b designation.
CURRENT GUIDANCE ON THE USE OF GEP TESTING
The panel reviewed current data available from the AJCC and the guidance for use of GEP by the NCCN and Melanoma Prevention Working Group (MPWG), two national groups that have weighed in on the use of this type of test. The NCCN guidelines note that 31-GEP may be considered for patients with confirmed diagnoses of melanoma as a part of the pathologic lab work.4 The guidance states that GEP may help “differentiate melanomas at low versus high risk for metastasis” and “may provide information on individual risk of recurrence, as an adjunct to standard American Joint Committee on Cancer (AJCC) staging.” It is important to note that 31-GEP testing is not intended to replace traditional clinicopathologic staging—it should be considered a supplemental objective source of information.
NCCN guidelines emphasize that cutaneous melanoma must be managed with respect to individual patient risk factors and preferences, as well as other considerations, which in some cases would emphasize the use of GEP testing.4 It should be emphasized that guidelines are suggestions that should be taken into strong consideration when making individual treatment considerations, but should allow for flexibility to tailor to individual circumstances. The NCCN guidelines state “...that all the recommended options are discussed with the patients. The clinical team should document the rationale for the clinical approach selected.”4
In a recently published consensus group article,31 members of the MPWG evaluated existing evidence supporting the utility of GEP testing in the management of patients with cutaneous melanoma in an effort to establish guidelines on its use. Based on their review of the literature, the MPWG members concluded that, though there are certain instances when GEP testing is helpful (e.g., in patients with Stage II or IIA disease), “additional studies” evaluating the validity and clinical applicability of GEP testing are needed before its integration into guidelines can be recommended. Unfortunately, the MPWG consensus group did not elaborate on how to effectively utilize the available test during this transitional period when additional research is being conducted. In addition, there are important limitations that naturally occur in a consensus statement of this type. For example, of the 195 melanoma experts invited to participate in the survey by MPWG, 78 responded to the first survey and 28 responded to both surveys, leaving some question about the majority opinion. In addition, “consensus” was defined as the majority opinion (50-percent agreement, or 39 experts). Finally, the majority of the surveyed sample were members of academia and therefore might not have been representative of community providers with experience using the test. Thus, the possibility of sampling error and potential bias in this consensus exists.31
In summary, as discussed previously, AJCC data demonstrate that stage-based prognosis has gaps that prevent optimization of care. NCCN and MPWG expert groups recognize that use of GEP testing may be warranted; however, no specific guidance has been provided for a rational approach to its use. For this reason, the authors of the current article discussed and reviewed their personal experiences with respect to use of the test and outlined a rationale for use that incorporates the current standards of care recommended by NCCN.
DISCUSSION
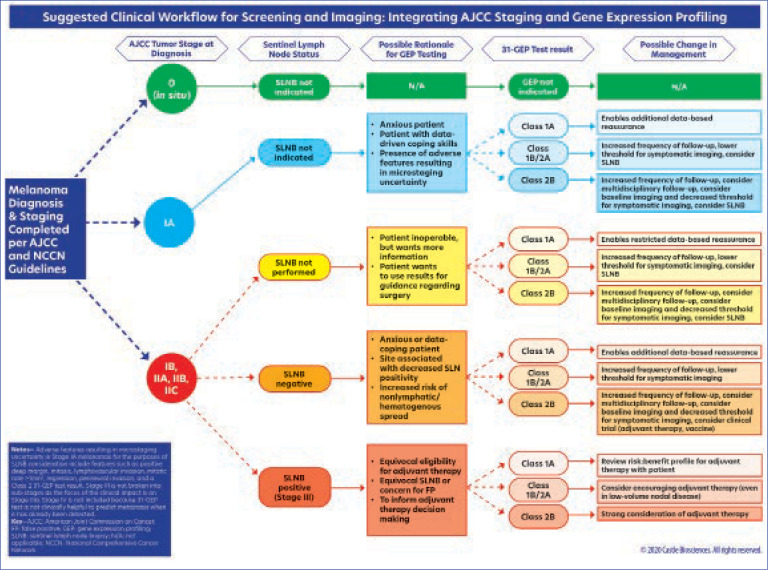
Following an in-depth review of SLNB- and GEP-based prognostic testing data, as well as a review of the national guidelines on GEP testing for melanoma, the authors of this article developed a clinical workflow (Figure 6) that provides ways in which the 31-GEP test can be used in alignment with existing NCCN guidelines. In particular, the authors wish to highlight the importance of discussing the use of the test (or the decision not to use the test) in the context of multidisciplinary care and with a multidisciplinary tumor board whenever possible. Importantly, there are no absolutes in risk assessment. Higher-risk patients may be stratified so that closer monitoring, including the possibility of regular imaging, can be provided by the multidisciplinary melanoma team. Lower-risk patients may also be routinely assessed according to their individual risk profiles. Early and objective identification of risk may improve patient adherence to the treatment plan, which could possibly have morbidity and mortality benefits.32 It is important to also recognize that the 31-GEP test is neither diagnostic nor definitive—it is intended to provide additional information that can be used to more completely risk-stratify a patient with melanoma, fortifying the clinical judgment of the dermatologist and enriching management options.
FIGURE 6.
A proposed clinical algorithm for implementing 31-GEP testing in different clinical situations within current AJCC staging and integrating it into the NCCN guidelines for cutaneous melanoma
Part of the rationale for use of GEP is based on the improvement of imaging techniques and technologies in recent years that can be applied to follow-up care. The main function of imaging is to provide an objective, high-resolution image to help determine if a tumor has penetrated the basement membrane.29 Many patients with metastatic melanoma are asymptomatic, and, thus, positron-emission computed tomography (PET-CT) imaging in high-risk patients may be particularly valuable in early detection of recurrence. Earlier detection of recurrence and better risk stratification could also guide medical oncology choices. In a study of patients with advanced melanoma who were treated with systemic therapy, the best response occurred in those patients with the lowest tumor burden and normal levels of lactate dehydrogenase.33 Conversely, there exists a benefit in the identification of the Class 1a/1b patients for whom imaging may be employed less frequently, based on their lower overall risk of recurrence.
Therapeutic options are also advancing rapidly in the management of cutaneous melanoma, and better risk stratification may help truncate time from diagnosis to treatment by more rapidly identifying the highest-risk patients. Biological risk stratification may also help identify patients who appear to be at low risk of metastases but are actually at high risk for metastases. Optimal care for patients with cutaneous melanoma involves the consideration of all risk factors and all treatment options when formulating individualized treatment plans. Integrating 31-GEP testing into risk stratification and prognoses of cutaneous melanoma can help to more accurately predict risk of metastasis and recurrence in patients with AJCC Stage I–III melanoma.
The suggested clinical workflow (Figure 6) has been designed to support compliance with the NCCN guidelines while enhancing the integration of 31-GEP testing into current standards of melanoma staging. The workflow begins with NCCN guidelines for patients with AJCC-staged melanoma. In this workflow, it is not recommended that GEP testing be performed on melanoma in situ, as this subgroup is not eligible for the test. The rationale for 31-GEP testing is different based on three staging groups: Stage IA, Stages IB– IIC, and Stage III.
Stage IA patients who are anxious, data-driven, and/or present with adverse features resulting in uncertain microstaging (e.g,. biopsies with a transected base, mitoses, lymphovascular invasion, tumor infiltrating lymphocytes) may benefit from the added information provided by the 31-GEP test. A GEP Class 1a result can reassure these patients that their tumor profile is consistent with the AJCC data for Stage IA and that SLNB is not indicated. If a Stage IA tumor has a Class 1b, 2a, or 2b profile, however, this suggests the tumor is biologically more aggressive and more aggressive follow-up and/or an SLNB may be warranted.
In cases of Stage IB–IIC melanoma, an SLNB will most likely have already been offered to the patient per NCCN guidelines; however, some of these patients may decline SLNB (e.g., due to anxiety about surgery or presence of comorbidities that contraindicate its use) or be undecided regarding undergoing the procedure, and 31-GEP testing may offer them helpful information. For example, a GEP test result of 1b, 2a, or 2b may help undecided patients determine whether SLNB is a surgery they wish to have and/or whether more aggressive follow-up for them is warranted. A Class 1a result might provide patients with reassurance and a basis for better analysis regarding the role of surgery in their situation. For the patients who do undergo SLNB and are node-negative, the 31-GEP test could be of benefit to those who are anxious or for whom the site of the procedure is associated with lower nodal positivity, as well as shed light on the potential for increased hematogenous spread. For node-negative patients who receive a Class 2b 31-GEP result, counseling regarding participation in adjuvant and/or vaccine trials may be offered.
In the Stage III (node-positive) population, the 31-GEP test may be used to inform adjuvant therapy decision-making, particularly in Stage IIIA disease and/or if the patient has equivocal eligibility for adjuvant therapy, equivocal SLNB results, or concern for false-positive SLNB results. In particular, 31-GEP testing may add value to the risk/benefit discussion clinicians have with these patients who are undecided about adjuvant therapy (e.g., due to concerns regarding the side-effect profile).
Special populations, such as pregnant patients, may also deserve special consideration for GEP testing, as may patients who hesitate to have surgery due to COVID-19-related concerns. Subclassifying patients with Stage IIB, IIC, or IIIA disease who are at greater or lesser risk of aggressive disease progression may help providers better triage patients for imaging/follow-up protocols and/or adjuvant therapies. In particular, for node-negative patients who are not eligible for adjuvant therapy, a high-risk 31-GEP result may be useful in prioritizing the referral of these patients for participation in clinical trials.
The key to appropriate use of 31-GEP testing is the incorporation of a shared decision-making model, implemented by the multidisciplinary team and the patient with melanoma, with the goal of educating the patient on the risks and benefits of treatment options so that the patient can make informed decisions. Shared decision making requires the clinician’s respect for each patient’s individual preferences and treatment objectives, which may not necessarily align with the preferences of the clinician or multidisciplinary team as a whole. This model has been shown to increase treatment adherence and patient satisfaction.34–36
Limitations. This article presents the consensus of a panel of melanoma experts with varying levels of experience in the clinical use of the 31-GEP test, who met to discuss how 31-GEP testing could most effectively be integrated into current clinical workflow for melanoma. The proposed clinical workflow is not based on a randomized clinical trial; rather, it is based on the panel’s review of relevant literature. The rationale and management opportunities outlined in this algorithm are based on the collective experience of the group using 31-GEP testing in the real world, and are presented as a reasonable and important intermediate form of guidance.
Many variables can affect outcomes in cutaneous melanoma, such as immune status and surgical care, and variables such as these have not been elucidated or incorporated into the proposed prognostic algorithms. Furthermore, while imaging is emerging as an important tool in melanoma assessment and management, the optimal imaging modality and frequency of imaging have not yet been determined. The role of careful clinical judgment must be emphasized, even when using an algorithmic guide. The population of patients with cutaneous melanoma is large and heterogeneous, and dermatologists must carefully consider the individual needs and wants of each patient when developing a treatment plan.
CONCLUSION
Early intervention is the goal for melanoma treatment, and better prognostic accuracy must be achieved. Dermatologists, who are on the front line for their patients in terms of melanoma diagnosis and management, may embrace new technology so that patients, regardless of risk status, can receive optimal and appropriate care. This panel recognizes that 31-GEP testing has value in conjunction with SLNB in the prognostication of patients diagnosed with melanoma. The 31-GEP test can further risk-stratify patients, identifying those at elevated or lower risk for both SLNB positivity and recurrence. The 31-GEP test is not intended to replace the current clinical pathologic staging or SLNB, but can be considered as an adjunctive tool to provide individualized information. The 31-GEP test has a robust and consistent body of evidence that supports its utility. From retrospective validity studies to prospective publications, results, to date, have been uniformly consistent, and several clinical impact studies have provided insight into how this test is changing management decisions in the US. The panel recommends that practitioners carefully consider the rationale for 31-GEP testing and utilize it when it permits better risk-stratification for patients with cutaneous melanoma. Clinical decisions should be guided by multidisciplinary discussions and a shared decision-making model with patients that is tailored to their individual needs and preferences. Additional prospective and retrospective studies are needed to expand the body of evidence supporting the use of 31-GEP testing in the management of melanoma.
FIGURE 7.
Forest plot of odds ratio and 95% confidence interval (CI) for A) recurrence, B) distant metastsis, C) overall survival, and D) sentinel lymph node biopsy (SLNB) positivity
Reproduced with permission. Litchman GH, Prado G, Teplit RWz, Rigel D. A systematic review and meta-analysis of gene expression profiling for primary cutaneous melanoma prognosis. SKIN: J Cutan Med. 2020;4(3):221–237
REFERENCES
- American Cancer Society site. Incidence of melanoma: cancer facts and figures 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf Accessed 8 Jan 2020.
- Amin MB, Edge SB, Greene FL, editors. 8th ed. Switzerland: Springer; 2017. AJCC Cancer Staging Manual. [Google Scholar]
- NIH. National Cancer institute site. https://seer.cancer.gov/statfacts/html/melan.html Surveillance, epidemiology, and end results program (SEER). Cancer stat facts: melanoma of the skin. Accessed 18 Aug 2020.
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Cutaneous Melanoma (Version 3.2020). https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf 18 May 2020. Accessed 31 Aug 2020.
- Zager JS, Gastman BR, Leachman S et al. Performance of a prognostic 31-gene expression profile in an independent cohort of 523 cutaneous melanoma patients. BMC Cancer. 2018;18(1):130. doi: 10.1186/s12885-018-4016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerami P, Cook RW, Wilkinson J et al. Development of a prognostic genetic signature to predict the metastatic risk associated with cutaneous melanoma. Clin Cancer Res. 2015;21(1):175–183. doi: 10.1158/1078-0432.CCR-13-3316. [DOI] [PubMed] [Google Scholar]
- Cook RW, Middlebrook B, Wilkinson J et al. Analytic validity of DecisionDx-Melanoma, a gene expression profile test for determining metastatic risk in melanoma patients. Diagn Pathol. 2018;13(1):13. doi: 10.1186/s13000-018-0690-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhaw BN, Zitelli JA, Brodland DG. Estimation of prognosis in invasive cutaneous melanoma: an independent study of the accuracy of a gene expression profile test. Dermatol Surg. 2018;0:1–7. doi: 10.1097/DSS.0000000000001588. [DOI] [PubMed] [Google Scholar]
- Keller J, Schwartz TL, Lizalek JM et al. Prospective validation of the prognostic 31-gene expression profiling test in primary cutaneous melanoma. Cancer Med. 2019;8(5):2205–2212. doi: 10.1002/cam4.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlipnik S, Carrera C, Boada A et al. Early outcome of a 31-gene expression profile test in 86 AJCC stage IB–II melanoma patients: a prospective multicentre cohort study. J Eur Acad Dermatol Venerol. 2019;33(5):857–862. doi: 10.1111/jdv.15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon LD, Gadzia JE, Davidson RS et al. Prospective, multicenter clinical impact evaluation of a 31-gene expression profile test for management of melanoma patients. SKIN J Cutan Med. 2018;2:111–121. [Google Scholar]
- Mirsky R, Prado G, Svoboda R et al. Management decisions made by physician assistants and nurse practitioners in cutaneous malignant melanoma patients: impact of a 31-gene expression profile test. J Drugs Dermatol. 2018;17(11):1220–1223. [PubMed] [Google Scholar]
- Schuitevoerder D, Heath M, Cook RW et al. Impact of gene expression profiling on decision-making in clinically node negative melanoma patients after surgical staging. J Drugs Dermatol. 2018;17(2):196–199. [PubMed] [Google Scholar]
- Svoboda RM, Glazer AM, Farberg AS, Rigel DS. Factors affecting dermatologists’ use of a 31-gene expression profiling test as an adjunct for predicting metastatic risk in cutaneous melanoma. J Drugs Dermatol. 2018;17(5):544–547. [PubMed] [Google Scholar]
- Greenhaw BN, Covington KR, Kurley SJ et al. Molecular risk prediction in cutaneous melanoma: a meta-analysis of the 31-gene expression profile prognostic test in 1,479 patients. J Am Acad Dermatol. 2020;83(3):745–753. doi: 10.1016/j.jaad.2020.03.053. [DOI] [PubMed] [Google Scholar]
- Berman B, Ceilley R, Cockerell C et al. Appropriate use criteria for the integration of diagnostic and prognostic gene expression profile assays into the management of cutaneous malignant melanoma: an expert panel consensus-based modified delphi process assessment. SKIN: J Cutan Med. 2019;3(5):291–306. [Google Scholar]
- Dubin DP, Dinehart SM, Farberg AS. Level of evidence review for a gene expression profile test for cutaneous melanoma. Am J Clin Dermatol. 2019;20(6):763–770. doi: 10.1007/s40257-019-00464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchman GH, Prado G, Teplit RW, Rigel D. A systematic review and meta-analysis of gene expression profiling for primary cutaneous melanoma prognosis. SKIN: J Cutan Med. 2020;4(3):221–237. [Google Scholar]
- Marchetti MA, Coit DG, Dusza SW et al. Performance of gene expression profile tests for prognosis in patients with localized cutaneous melanoma: a systematic review and meta-analysis. JAMA Dermatol. 2020;156(9):953–962. doi: 10.1001/jamadermatol.2020.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton DL, Thompson JF, Cochran AJ et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370:599–609. doi: 10.1056/NEJMoa1310460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton DL, Thompson JF, Cochran AJ et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–1317. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- Gastman BR, Gerami P, Kurley SJ et al. Identification of patients at risk of metastasis using a prognostic 31-gene expression profile in subpopulations of melanoma patients with favorable outcomes by standard criteria. J Am Acad Dermatol. 2019;80(1):149–157. doi: 10.1016/j.jaad.2018.07.028. [DOI] [PubMed] [Google Scholar]
- Whiteman DC, Baade PD, Olsen CM. More people die from thin melanomas (<1mm) than from thick melanomas (>4mm) in Queensland, Australia. J Invest Dermatol. 2015;135(4):1190–1193. doi: 10.1038/jid.2014.452. [DOI] [PubMed] [Google Scholar]
- Shaikh WR, Dusza SW, Weinstock MA. Melanoma thickness and survival trends in the United States, 1989 to 2009. J Natl Cancer Inst. 2015;108(1):djv294. doi: 10.1093/jnci/djv294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerami P, Cook RW, Russell MC et al. Gene expression profiling for molecular staging of cutaneous melanoma in patients undergoing sentinel lymph node biopsy. J Am Acad Dermatol. 2015;72(5):780–785. doi: 10.1016/j.jaad.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Marks E, Caruso HG, Kurley SJ et al. Establishing an evidence-based decision point for clinical use of the 31-gene expression profile test in cutaneous melanoma. SKIN J Cutan Med. 2019;3(4):239–249. [Google Scholar]
- Vetto JT, Hsueh EC, Gastman BR et al. Guidance of sentinel lymph node biopsy decisions in patients with T1–T2 melanoma using gene expression profiling. Future Oncol. 2019;15(11):1207–1217. doi: 10.2217/fon-2018-0912. [DOI] [PubMed] [Google Scholar]
- Hsueh EC, DeBloom J, Lee J et al. Interim analysis of survival in a prospective, multi-center registry cohort of cutaneous melanoma tested with a prognostic 31-gene expression profile test. J Hematol Oncol. 2017;10(152):Epub 29. doi: 10.1186/s13045-017-0520-1. August 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlipnik S, Moreno-Ramírez D, Carrera C et al. Cost-effectiveness analysis of imaging strategy for an intensive follow-up of patients with American Joint Committee on Cancer Stage IIB, IIC, and III malignant melanoma. Br J Dermatol. 2019;180(5):1190–1197. doi: 10.1111/bjd.16833. [DOI] [PubMed] [Google Scholar]
- Farberg AS, Glazer AM, White R et al. Impact of a 31-gene expression profiling test for cutaneous melanoma on dermatologists’ clinical management decisions. J Drugs Dermatol. 2017;16(5):428–431. [PubMed] [Google Scholar]
- Grossman D, Okwundu N, Bartlett EK et al. Prognostic gene expression profiling in cutaneous melanoma: identifying the knowledge gaps and assessing the clinical benefit. JAMA Dermatol. 2020;156(9):1004–1011. doi: 10.1001/jamadermatol.2020.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe C, Peris K, Hauschild A et al. Diagnosis and treatment of melanoma: European consensus-based interdisciplinary guideline. Eur J Cancer. 2010;46(2):270–283. doi: 10.1016/j.ejca.2009.10.032. [DOI] [PubMed] [Google Scholar]
- Schadendorf D, Wolchok JD, Hodi FS et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized Phase II and III trials. J Clin Oncol. 2017;35(34):3807–3814. doi: 10.1200/JCO.2017.73.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwyn G, Frosch D, Thomson R et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361–1367. doi: 10.1007/s11606-012-2077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwyn G, Frosch DL, Kobrin S. Implementing shared decision-making: consider all the consequences. Implement Sci. 2016;11:114. doi: 10.1186/s13012-016-0480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D, Hill S, McCaffery K, Boland L, Lewis KB, Horvat L. Shared decision making interventions: theoretical and empirical evidence with implications for health literacy. Stud Health Technol Inform. 2017;240:263–283. [PubMed] [Google Scholar]