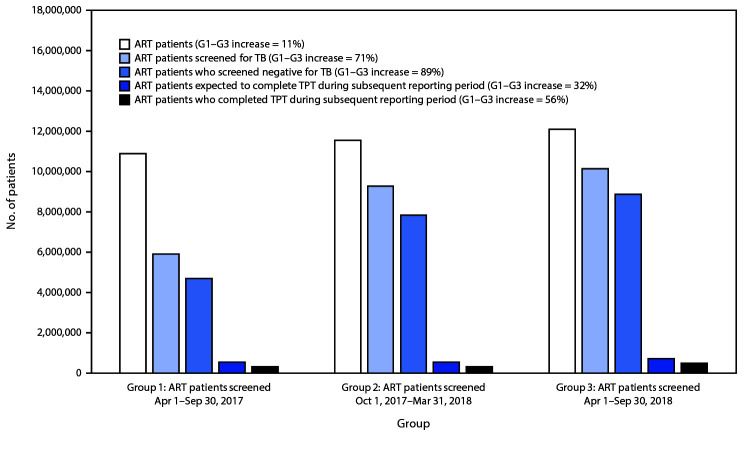
FIGURE 1.
Tuberculosis (TB) screening and TB preventive treatment (TPT) indicators*,†,§ for persons living with human immunodeficiency virus (HIV) infection receiving antiretroviral therapy (ART patients) — 16 PEPFAR-supported countries,¶ 2017–2019
Abbreviations: G = group; PEPFAR = U.S. President’s Emergency Plan for AIDS Relief.
* Number of ART patients reports a snapshot at the end of the reporting period, accounting for net gains (e.g., new HIV diagnoses) and losses (e.g., deaths and patients lost to follow-up).
† The South African National Department of Health (NDOH) receives PEPFAR support for health system strengthening and other non–patient care activities. NDOH annually reports ART patients in its care but does not report TPT initiation or completion. Therefore, ART patients reported by NDOH were subtracted from South Africa totals in period 1 (779,313) and in period 3 (900,463) to include data reported only by South African partners that received PEPFAR support for delivering health care services.
§ Number of ART patients expected to complete TPT approximates number of TPT initiations. If the patient is prescribed 6 months of daily isoniazid, the patient is expected to complete treatment during the reporting period subsequent to that of initiation. Otherwise, if prescribed a shorter rifamycin-based course, the patient might be expected to complete treatment during the initiation reporting period.
¶ Cameroon, Democratic Republic of the Congo, Eswatini, Ethiopia, Haiti, Kenya, Lesotho, Mozambique, Namibia, Nigeria, South Africa, Tanzania, Uganda, Vietnam, Zambia, and Zimbabwe.