Abstract
Treatment-emergent central sleep apnea (TECSA) is a specific form of sleep-disordered breathing, characterized by the emergence or persistence of central apneas during treatment for obstructive sleep apnea. The purpose of this review was to summarize the definition, epidemiology, potential mechanisms, clinical characteristics, and treatment of TECSA. We searched for relevant articles up to January 31, 2020, in the PubMed database. The prevalence of TECSA varied widely in different studies. The potential mechanisms leading to TECSA included ventilatory control instability, low arousal threshold, activation of lung stretch receptors, and prolonged circulation time. TECSA may be a self-limited disorder in some patients and could be resolved spontaneously over time with ongoing treatment of continuous positive airway pressure (CPAP). However, central apneas persist even with the regular CPAP therapy in some patients, and new treatment approaches such as adaptive servo-ventilation may be necessary. We concluded that several questions regarding TECSA remain, despite the findings of many studies, and it is necessary to carry out large surveys with basic scientific design and clinical trials for TECSA to clarify these irregularities. Further, it will be vital to evaluate the baseline demographic and polysomnographic data of TECSA patients more carefully and comprehensively.
Keywords: Central sleep apnea, Epidemiology, Risk factors, Treatment
Introduction
Treatment-emergent central sleep apnea (TECSA, formerly complex sleep apnea) describes the appearance of central sleep apnea (CSA) and/or hypopnea while undergoing treatment for obstructive sleep apnea (OSA).[1] TECSA was observed in some patients who primarily had OSA or mixed apneas after the significant resolution of the obstructive events by treatment with a positive airway pressure (PAP) device without a back-up rate.[2,3] TECSA has recently been observed after various treatment modalities for OSA, including the use of a mandibular advancement device (MAD), maxillomandibular advancement surgery, sinus and nasal surgery, and tracheostomy.[4–9] In some TECSA cases, occurrence of CSA events during initial continuous positive airway pressure (CPAP) titration are transient and they may resolve spontaneously with chronic CPAP therapy.[10] However, some central apneas persist even with regular CPAP therapy.[10] The development of TECSA has affected the effectiveness of OSA treatment and patients’ compliance with it.[2,11] Therefore, in order to analyze this condition properly, several studies on TECSA have recently been conducted. However, there is still some controversy regarding the prevalence of TECSA and the optimal treatment method for it, and its importance and significance have not yet been fully established. This review aimed to summarize the definition, epidemiology, potential mechanisms, and clinical characteristics of TECSA, and review the treatment methods for it.
Definition
TECSA refers to a specific form of sleep-disordered breathing, characterized by the emergence or persistence of central apneas during treatment for OSA such as using PAP therapy.[1] Gilmartin et al[12] first described this phenomenon using the new term “complex sleep-disordered breathing.” Then, Morgenthaler et al[13] termed this type of sleep-disordered breathing “complex sleep apnea syndrome (CompSAS).” The International Classification of Sleep Disorders-third edition introduced the term “TECSA” as a new name for this phenomenon; it is defined as the presence of primary OSA at the initial diagnostic sleep study, significant resolution of obstructive events with CPAP titration but emergence or persistence of central events during PAP treatment with a central apnea index (CAI) ≥5/h, greater than 50% of events being central, and the symptoms cannot be better explained by another CSA disorder.[1] However, new-onset CSA has also been reported during other non-CPAP treatments for OSA, including surgery and the use of oral appliances. Goldstein and Kuzniar[9] reported a 43-year-old man with mild OSA developed CSA after endoscopic sinus and nasal surgery for nasal obstruction. The patient's nasal congestion improved, but night-time sleep fragmentation and excessive daytime sleepiness both worsened 4 months after surgery in this case; he had not gained any weight and did not have any cardiopulmonary symptoms. Some studies have reported that other surgical interventions, such as tracheostomy and maxillomandibular advancement, resulted in the appearance of CSA post-operatively.[8,14,15] Mohan et al[4] noted a patient with moderate OSA who used a MAD initially, had CSA. The spontaneous resolution of MAD-emergent CSA was observed in this patient 1 year after MAD treatment. There have been three reports on the occurrence of similar MAD-emergent CSA during treatment with a MAD.[5–7] Therefore, the definition of TECSA should refer to the phenomenon of transient and/or persistent CSA after not only CPAP treatment but also all kinds of therapy for OSA.
Epidemiology
With the increasing awareness of TECSA, more studies have evaluated its prevalence and natural course. However, the prevalence of TECSA appears to vary widely among different studies ranging from 0.56% to 20.3%.[3,16–22] [Table 1]. The large variation in the prevalence of TECSA may be related to differences in the methodologies of these studies, including protocol designs (retrospective or prospective study), study populations selected sample sizes, inclusion criteria for the TECSA patients, procedures of the studies (split-night titration or full-night titration), and different follow-up periods.
Table 1.
Prevalence of treatment-emergent central sleep apnea in patients with obstructive sleep apnea in previous studies.
| Studies | Reported time (year) | Study location | Study design | Type of sleep study | Study population | Prevalence of TECSA |
| Morgenthaler, et al[13] | 2006 | USA | Retrospective | SN | 223 patients with sleep-related breathing disorders | 15% (34/223) |
| Pusalavidyasagar, et al[52] | 2006 | USA | Retrospective | SN | 167 patients with OSA | 20.3% (34/167) |
| Lehman, et al[53] | 2007 | Australia | Retrospective | FN/SN | 99 patients with OSAH | 13.1% (13/99) |
| Dernaika, et al[35] | 2007 | USA | Cross-sectional | SN | 116 patients with OSA (AHI ≥20/h) | 19.8%(23/116) |
| Kuzniar, et al[19] | 2008 | USA | Retrospective | SN | 116 patients with OSA | 6.5% (13/200) |
| Endo, et al[20] | 2008 | Japan | Retrospective | FN | 1312 patients with SAS (AHI ≥20/h) | 5.0% (66/1312) |
| Javaheri, et al[18] | 2009 | USA | Retrospective | FN | 1286 patients with OSA | 6.5% (84/1286) |
| Yaegashi, et al[55] | 2009 | Japan | Retrospective | FN | 297 patients with OSA (AHI ≥20/h) | 5.7% (17/297) |
| Cassel, et al[21] | 2011 | Germany | Prospective | FN | 675 patients with OSA | 12.2% (82/675) |
| Pagel, et al[26] | 2011 | USA | Retrospective | SN | 150 OSA patients with AHI >15 living at an altitude of 1421 m; | 10.6% (16/150) at an altitude of 1421 m; |
| 150 OSA patients with AHI >15 living at an altitude of 1808 m; | 22% (33/150) at an altitude of 1808 m; | |||||
| 142 OSA patients with AHI >15 living at an altitude of 2165 m | 38.7% (55/142) at an altitude of 2165 m | |||||
| Bitter, et al[24] | 2011 | Germany | Prospective | FN | 192 patients with CHF (LVEF ≤45%, NYHA class ≥2) and OSA (AHI ≥15) | 18% (34/192) |
| Westhoff, et al[16] | 2012 | Germany | Prospective | FN | 1776 patients with OSA (AHI ≥15) and normal BNP levels | 0.56% (10/1776) |
| Neu, et al[23] | 2015 | Belgium | Retrospective | FN | 263 patients with OSA (AHI >20/h and ArI >30) | 9.1% (24/263) |
| Zapata, et al[25] | 2015 | Colombia | Prospective | FN | 988 patients with OSA (AHI >15/h) | 11.6% (115/988) |
| Moro, et al[54] | 2016 | USA | Retrospective | FN/SN | 728 OSA patients who underwent PAP titration (n = 422 split-night; n = 306 two-night) | 24.2% of SN-PSG;11.4% of FN-PSG. |
| Liu, et al[2] | 2017 | USA, Germany, Australia, France | Retrospective | NA | 133,006 OSA patients used CPAP for ≥90 days and had ≥1 day with the use of ≥1 h in week 1 and week 13 (a telemonitoring device database) | 3.5% |
Data are presented as % (n/N). TECSA: Treatment-emergent central sleep apnea; SN: Split night titration study; FN: Full night titration study; NA: Not applicable; OSA: Obstructive sleep apnea; OSAH: Obstructive sleep apnea-hypopnea; SAS: Sleep apnea syndrome; AHI: Apnea-hypopnea index; CHF: Congestive heart failure; LVEF: Left ventricular ejection fraction; NYHA: New York Heart Association; BNP: Brain natriuretic peptide; ArI: Arousal index; PAP: Positive airway pressure; CPAP: Continuous positive airway pressure; PSG: Polysomnography.
Most studies on the prevalence of TECSA are retrospective studies, and they often have limitations such as selection bias. In addition, the sample sizes in most studies were generally small. A systematic review identified nine studies on the prevalence of TECSA.[3] Seven of the nine studies were retrospective in design, whereas one was a prospective observational study and another was a cross-sectional study. Three of the nine studies included follow-up observation. The sample size of the subjects with OSA in these studies ranged from 99 to 1312. The authors reported that the aggregate point prevalence of TECSA was about 8%. In this review, the estimated range of the prevalence of TECSA in patients with untreated OSA was from 5% to 20%. Recently, in a large sample study of real-life population-based data, Liu et al[2] analyzed telemonitoring device data for the presence/absence of emergent CSA at baseline (week 1) and week 13. Patients (133,006) used CPAP for ≥90 days and had ≥ 1 day of ≥ 1 h use in week 1 and week 13. The proportion of patients with CSA in week 1 or week 13 was 3.5%; of these, CSA was transient, persistent, or emergent in 55.1%, 25.2%, and 19.7%, respectively.
The differences in the procedures used in studies on TECSA may be responsible for the differences in the recorded prevalence of TECSA, especially for split-night or full-night studies. Out of the aforementioned nine studies, four studies used full-night CPAP titration, four studies used the titration portion of a split-night study, and one study included subjects in both titration and split-night study groups. For studies that used only full night titration, the reported prevalence of TECSA ranged from 5.0% to 12.1% whereas it was between 6.5% and 20.3% for studies that used split-night polysomnogram. The prevalence of TECSA tended to be higher for split-night studies compared with full-night titration studies.[3] Neu et al[23] performed a retrospective cohort study of OSA patients using auto-titrating CPAP; among the 263 included subjects, 24 patients presented with complex sleep apnea at a CPAP trial, resulting in a prevalence of 9.1% (5.6%–12.6%, 95% confidence interval). The authors believed that auto-titration procedures may be more likely to induce CSA in certain patients; however, a controlled trial with manual titration or auto-titration was not performed in this study.
The inclusion criteria for the subjects in the TECSA prevalence studies varied, thus, the reported prevalence of TECSA varied as well, especially in studies that involved specific populations. For example, Bitter et al[24] described a high prevalence (18%) of CSA in OSA patients with congestive heart failure (CHF) (left ventricular ejection fraction ≤45% and New York Heart Association Class ≥2). On the contrary, Westhoff et al[16] reported that the prevalence of CSA or persisting CSA in patients with OSA and normal brain natriuretic peptide levels who were receiving CPAP therapy was low (0.56% and 1.57%, respectively). Regional differences in the populations evaluated in these studies should be considered as well. In a study based on OSA patients living in high altitude areas (2640 m), the prevalence of CSA was 11.6%, which was intermediate compared to the prevalence reported for patients living in areas with lower altitudes.[25] However, Pagel et al[26] analyzed OSA patients living in areas at three different altitudes above sea level (1421, 1808, and 2165 m, respectively), and reported that as altitude increases, central apnea becomes more frequent (10.6%, 22%, and 38.7%, respectively). Miao ZB et al[27] also demonstrated that the prevalence of TECSA increases as altitudes rise.
Mechanisms
TECSA is a non-hypercapnia-induced central sleep-disordered breathing. One critical factor that is considered in the cessation of airflow during sleep is the concept of the apnea threshold (AT) of measured partial pressure of carbon dioxide in arterial blood (PaCO2).[28,29] AT is an individual's set value of PaCO2. If the PaCO2 value falls below the AT during sleep, a CSA event will occur, and when the PaCO2 value rises above this threshold, respiratory airflow recovers.[28,29] More important than AT is the difference between eupneic PaCO2 and AT.[28,29] It is important to note that the PaCO2-AT difference is not fixed and can vary with the ventilatory drive. Individuals with narrower PaCO2-AT differences are predisposed to CSA.[28,29]
The physiological mechanism that leads to the occurrence of TECSA in OSA patients exposed to CPAP or other therapies is not well established. To date, several possible mechanisms including ventilatory control instability (high loop gain),[30–32] low arousal threshold,[33] activation of lung stretch receptors,[34,35] and prolonged circulation time,[36] have been suggested [Figure 1].
Figure 1.
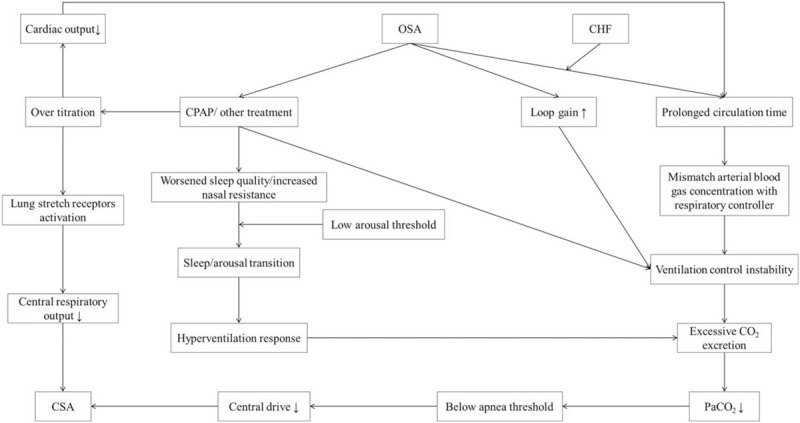
Schematic representation of potential pathophysiological mechanisms of treatment-emergent central sleep apnea in patients with obstructive sleep apnea. CHF: Congestive heart failure; CO2: Carbon dioxide; CPAP: Continuous positive airway pressure; CSA: Central sleep apnea; OSA: Obstructive sleep apnea; PaCO2: Partial pressure of carbon dioxide in arterial blood.
Ventilatory control instability
A previous study reported that severe OSA patients showed a high “loop gain” in their ventilatory control system, which is a strong predictor of ventilatory instability.[37] The loop gain is determined by the plant gain and controller gain. The plant gain is the ability of the lungs and respiratory muscles to increase ventilation, and the controller gain is the change in ventilation induced by a change in PaCO2 level.[28] Individuals with high loop gain may be at risk of having unstable breathing patterns because they “over-respond” to small changes in chemical stimuli[38]; for example, a small increase in PaCO2 can lead to greater hyperventilation in OSA patients with high loop gain compared with someone with low loop gain. This increased ventilation will continue until the resultant reduction in PaCO2 is detected. When the PaCO2 level falls below an individual's set value (AT), central hypoventilation or potential apnea will occur.[28] Ventilation will resume after the PaCO2 level has risen above that threshold. CPAP or other treatments for OSA can intermittently decrease the PaCO2 level to a value below the AT, which may explain the occurrence of TECSA in OSA.[3] Stanchina et al[39] reported that after 1 month of CPAP, patients with persistent CSA would have a higher loop gain on their CPAP titration night than patients with resolved apneas. The authors also suggested that loop gain measurement may enable a priori determination of those who need alternative modes of PAP therapy. All these points further support the notion that ventilatory control instability may be an important physiological mechanism for the occurrence of TECSA in OSA.
Low arousal threshold
The arousal threshold in OSA patients is defined as the inspiratory pressure generated just before arousal at the end of obstructive events.[30] Currently, a low respiratory arousal threshold is known as one of the pathophysiological phenotypes in some OSA patients.[40] Micro electroencephalographic arousals are often observed in OSA patients.[40] The frequent changes from sleep to arousal and from arousal to sleep due to low arousal threshold can cause instability in the ventilatory control system during sleep, especially non-rapid eye movement sleep.[41–48] Once ventilation increases, the subsequent ventilatory overshoot can decrease the PaCO2 level to a value below the AT, resulting in the emergence of central apnea.[41]
In addition, the worsened sleep quality[34,41] and increased nasal resistance[34,49] caused by CPAP may be related to the frequent arousals experienced by some OSA patients with low arousal threshold, which could presumably contribute to an increase in central apneas.
Activation of lung stretch receptors
Expansion of lung volumes induced by over-titration of CPAP may lead to activation of stretch receptors in the lungs; the receptors then send signals via the vagal nerve fibers to the respiratory center, which then inhibits the central respiratory output. Consequently, an interruption of inspiration will occur. This mechanism can also protect the lungs against overexpansion.[35]
Prolonged circulation time
Circulation delay in OSA patients with CHF can lead to a mismatch in arterial blood gas concentration with the respiratory controllers[38,50,51]; this may explain why OSA patients with CHF are more likely to develop TECSA. Moreover, CPAP over-titration could reduce cardiac output, which may further contribute to ventilatory control instability due to prolonged circulation time.
All these physiological mechanisms may be clinically significant, therefore, fully identifying their interaction and their role in triggering TECSA is vital to enable practitioners to select the most appropriate clinical therapies for this condition.
Risk Factors and Clinical Characteristics
Certain risk factors, including demographic data, clinical information, and polysomnographic characteristics, are associated with a higher prevalence of TECSA [Table 2]. Some studies identified several clinical risk factors for TECSA in OSA patients. As these studies reported, older age,[21] male,[13,25,52–54] lower body mass index,[19,52] comorbid conditions (especially coronary artery disease, hypertension and CHF, atrial fibrillation, and stroke),[23,25,53,54] medications (chronic opiate use),[18,54] certain polysomnographic parameters at the time of diagnostic polysomnography (PSG) study (such as higher baseline apnea-hypopnea index [AHI] [10,18,20,21,23,25,53] and CAI,[11,18,21,23,25,52,53] higher baseline arousal index,[10,53] and an increase in CAI in non-rapid eye movement supine sleep[55]), and titration factors (such as higher residual AHI,[10] rapid or excessively high titration,[3,56] excessive air leak,[3,56] lower total sleep time,[10] lower sleep efficiency,[10] and use of bilevel positive airway pressure (BiPAP) in their titration studies[31]) were associated with a higher prevalence of TECSA in OSA patients than those in matched control subjects. Lei F et al[57] reported that higher baseline mixed sleep apnea, especially in non-rapid eye movement sleep, was related to a higher incidence of TECSA in OSA patients. Herkenrath S et al[58] compared mixed apnea metrics during diagnostic PSG in OSA patients with or without TECSA, and found that those with TECSA had longer apneic duration, more frequent arousals, and shorter ventilatory duration, consistent with higher ventilatory control instability. However, there is still no consensus on the risk factors for TECSA, and the same risk factors are not always identified in the different studies.
Table 2.
Potential risk factors for treatment-emergent central sleep apnea in patients with obstructive sleep apnea in previous studies.
| Studies | Older age | Male | Cardiovascular history | Lower BMI | Intake of opioids (/narcotic use) | Higher baseline CAI | Higher baseline AHI | Higher baseline ArI | Higher baseline ESS | Higher CAI in NREM supine sleep | Higher hypercapnic ventilatory response |
| Morgenthaler, et al[13] | − | + | − | − | NM | − | − | − | − | NM | NM |
| Pusalavidyasagar, et al[52] | − | + | − | + | NM | + | − | NM | − | NM | NM |
| Lehman, et al[53] | − | + | + | NM | − | + | + | + | NM | NM | NM |
| Dernaika, et al[35] | − | NM | − | − | NM | − | − | − | − | NM | NM |
| Kuzniar, et al[19] | − | − | NM | + | NM | NM | NM | NM | + | NM | NM |
| Endo, et al[20] | − | − | − | − | − | NM | + | − | NM | NM | NM |
| Javaheri, et al[18] | − | − | − | − | + | + | + | − | − | NM | NM |
| Yaegashi, et al[55] | − | − | − | − | NM | − | − | NM | NM | + | NM |
| Cassel, et al[21] | + | − | − | − | − | + | + | − | − | NM | NM |
| Bitter, et al[24] | − | − | − | − | NM | NM | − | NM | NM | NM | + |
| Neu, et al[23] | − | − | + | − | − | + | + | − | − | NM | NM |
| Zapata, et al[25] | − | + | + | − | NM | + | + | NM | − | NM | NM |
| Moro, et al[54] | − | + | +∗ | − | +† | +‡ | − | NM | NM | NM | NM |
∗Self-reported history of stroke; †Self-reported narcotics were a positive predictor of treatment-emergent central sleep apnea only for spilt-night-PSG patients; ‡The central apnea index during the diagnostic recording predicted treatment-emergent central sleep apnea only for full-night-PSG patients. +: Data are statistically significant; −: Data are not statistically significant. BMI: Body mass index; CAI: Central apnea index; AHI: Apnea-hypopnea index; ArI: Arousal index; ESS: Epworth sleepiness scale; NREM: Non-rapid eye movement; NM: Not mentioned.
Regarding demographic characteristics, most of the risk factors reported in these studies indicated that patients with TECSA are more likely to be male, older, and relatively less severely obese. However, the authors of these studies[13,59] did not state whether they observed any significant age differences between patients who developed TECSA and patients who did not.
Regarding comorbid conditions, TECSA is more common in OSA patients with cardiovascular and cerebrovascular diseases, especially CHF. The high prevalence (18%) of CSA in OSA patients with CHF and the low prevalence (0.56%) of CSA in OSA patients without evidence of heart failure indicate that CHF is a potentially important risk factor for TECSA. Lehman et al[53] found that a significantly greater number of patients with TECSA had CHF and ischemic heart disease, whereas Cassel et al[21] found a similar trend that did not reach statistical significance. Self-reported cardiac disease (coronary artery disease, CHF, or atrial fibrillation) did not show any correlation with TECSA in a retrospective study[54]; the reason for the difference between the results of this study and that of other studies may be that the medical history was obtained by self-report. Moreover, Kuźniar et al[60] found that although most patients with CompSAS have cardiac comorbidities, about one-third of patients in their study did not have any risk factors for CompSAS before sleep testing.
In addition, it should be noted that TECSA is common among opioid users since patients who are long-term opioid users are susceptible to CSA.[61] Despite this, in this patient population opioid use has not been identified as a risk factor for CompSAS.[62–64]
Treatment
Specific intervention for TECSA: is it necessary?
As some authors reported, TECSA may be a self-limited disorder in some patients. Some central respiratory events are transient and could spontaneously resolve over time with ongoing treatment with CPAP therapy.[39] In a case report, Mohan et al reported that spontaneous resolution of MAD-emergent CSA was seen more than 1 year after the maintenance of MAD treatment in a patient with moderate OSA who refused PAP therapy; the authors suggested that the key to the resolution of central respiratory events should be strict adherence to effective therapy.[4] However, central apneas still persist in some patients even with regular CPAP therapy. Hence, there is still some controversy about the optimal method for treating TECSA.
PAP treatment
CPAP
As previously mentioned, CSA will spontaneously resolve with chronic CPAP therapy in some TECSA patients. If a patient is tolerating CPAP well, he/she may not need to change his treatment regimen.[28]
Some studies have shown that CSA events naturally disappear after a few months with the maintenance of CPAP treatment.[18,21,52] In a large retrospective cohort study, Javeheri et al[18] reported that CompSAS of 33 out of 42 patients resolved with a second CPAP titration 5 to 6 weeks after CPAP treatment. The possible explanation for this is that CPAP treatment can resolve CSA events over time by improving the stability of the ventilatory control system[39] and lung volume.[65] Previous studies also reported that ventilatory response to hypoxia and hypercapnia decreased markedly after CPAP treatment in OSA patients without heart failure.[49,66]
However, CPAP seems to be ineffective in some patients. Correia et al[67] compared the clinical impact of adaptive servo-ventilation (ASV) with other forms of PAP in treating patients with TECSA, CSA, and Cheyne-Stokes respiration (CSR), and found that some CSA events persisted even with regular CPAP therapy. Fourteen out of 54 patients with an initial CSA diagnosis on CPAP titration night continued to meet the criteria for CSA at follow-up, and 16 of 382 patients (about 4%) not initially diagnosed with CSA developed novel CSA after 3 months of CPAP therapy.[21]
Several studies suggested that there is a risk of poor compliance and/or therapy termination in patients who had a bad initial experience with CPAP due to TECSA.[17,18,59] Moreover, there are very few available methods for predicting which patient can naturally resolve the central respiratory events of TECSA with CPAP treatment over time. Hence, it is necessary for TECSA patients undergoing CPAP therapy to receive follow-up assessments more frequently.
BiPAP with a back-up respiratory rate
BiPAP with a back-up respiratory rate, for example, BiPAP spontaneous/timed (BiPAP-S/T), can be an effective alternative for treating TECSA in patients who do not respond to CPAP.[68] BiPAP-S/T is a kind of non-invasive ventilation mode that provides expiratory positive airway pressure to eliminate obstructive events and inspiratory positive airway pressure and back-up ventilation rate to decrease hypoventilation.[17,22] Moreover, it can resolve CSA events by forcing breath (timed breath) when CSA occurs. Some pieces of evidence have confirmed that CPAP is inferior to bilevel devices with a back-up respiratory rate in treating patients with CompSAS.[24,68–73] Although the available data are limited, several studies suggest that both BiPAP and ASV improve CSA.
ASV
ASV is a novel ventilatory mode, which can provide a dynamic adjustment of inspiratory pressure support and a back-up respiratory rate.[67] ASV can alter pressure support accordingly based on a target minute ventilation calculated by measuring or estimating the respiratory output of the patient, in order to avoid central hypopnea/apnea events due to hyperventilation and associated hypocapnia[67]; therefore, this device can provide more stabilized ventilation.
ASV can be used for patients whose TECSA does not improve after continued use of CPAP or BiPAP without a back-up rate. Many studies have confirmed its effectiveness in treating patients with CSA.[33,68,69] Correia et al compared the clinical impact of ASV with other forms of PAP in treating patients with TECSA, CSA, and CSR.[67] The results demonstrated that ASV is an effective treatment method for treating patients with TECSA, CSA, and CSR since it significantly decreased residual AHI. Moreover, the compliance rate was high, and long-term tolerance was also excellent. In a prospective randomized controlled trial, 30 patients who developed CSA during CPAP treatment were randomized to non-invasive positive pressure ventilation or a servo-ventilation group. After 6 weeks of treatment, it was found that servo-ventilation treated respiratory events more effectively than non-invasive positive pressure ventilation.[73] Similarly, Morgenthaler et al[74] conducted a prospective, randomized, single-blind, multi-center trial to compare the efficacy of ASV to that of CPAP in resolving CompSAS over 90 days. They found lower residual AHI among patients treated with ASV compared with those on CPAP (4.7 ± 8.1 [central 1.1 ± 3.7] vs. 14.1 ± 20.7 [central 8.8 ± 16.3], P < 0.001); 89.7% of the patients treated with ASV achieved AHI <10/h, whereas only 64.5% of the patients treated with achieved AHI <10/h. Several studies have analyzed the role of ASV in the treatment of TECSA and their results have shown that ASV is an excellent treatment option.[67–70,74,75]
Clearly, ASV treatment for TECSA may effectively decrease residual CSA events, improve the symptoms of this disease, and increase adherence to therapy. However, this ventilation mode is associated with more expensive and complicated devices. Insufficient available data support ASV as a very early intervention strategy for the treatment of TECSA. Presently, continued use of CPAP may be the first treatment option when initial TECSA is noted on titration night. Subsequently, the TECSA patient should undergo follow-up assessment earlier and more frequently. At the follow-up session, the mode of PAP should be changed to either BiPAP with a back-up rate or ASV if central apneas do not naturally resolve [Figure 2].
Figure 2.
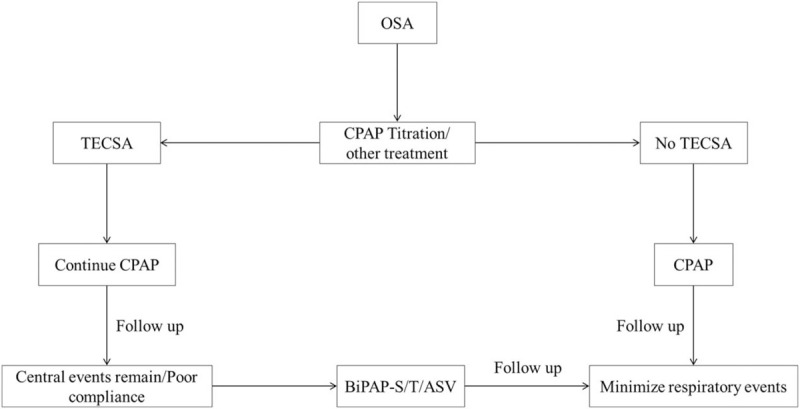
Flow diagram of treatment of treatment-emergent central sleep apnea in patients with obstructive sleep apnea. ASV: Adaptive servo-ventilation; BiPAP-S/T: Bilevel positive airway pressure-spontaneous/timed; CPAP: Continuous positive airway pressure; OSA: Obstructive sleep apnea; TECSA: Treatment-emergent central sleep apnea.
Medications
Medications could be selected to improve ventilatory control stability or elevate the arousal threshold for TECSA patients, which may be a supplement to PAP therapy. For example, acetazolamide, a carbonic anhydrase inhibitor, can modulate loop gain. Glidewell et al described a case of successful control of CompSAS with CPAP and acetazolamide in a 41-year-old white woman on long-term opioid therapy.[76] This case suggested that acetazolamide may be an effective adjunct to PAP therapy for the treatment of TECSA in patients on long-term opioid therapy. Similarly, some studies reported that both trazodone[77] and eszopiclone[78] increased the respiratory arousal threshold of OSA patients with a low arousal threshold. Hence, these medications could possibly be a new method for the treatment of TECSA. It is necessary to conduct large sample clinical trials to confirm the effectiveness and safety of such therapeutic options.
Oxygen therapy
Oxygen supplementation may be helpful in the treatment of TECSA since oxygen can significantly reduce the loop gain in OSA patients with a high loop gain.[79] Short-term studies have shown that oxygen supplementation during sleep reduces the number of central respiratory events in some CHF-CSA patients.[80,81] Oxygen therapy combined with any PAP modality may be a good option, especially for a TECSA patient that needs nocturnal oxygen therapy.[82] However, more evidence is needed before recommending oxygen therapy as a viable treatment option for TECSA.
Carbon dioxide (CO2) supplementation
Since the excessive excretion of CO2 is linked to the pathophysiology of TECSA, increasing PaCO2 levels and keeping the PaCO2 value above the AT may abolish CSA in TECSA patients. Hence, some studies involved the use of CO2 supplementation for the treatment of TECSA. In a small sample size study, Thomas et al[83] observed that inhaling a low concentration of CO2 via a PAP gas modulator resulted in a quick abolition of sleep-disordered breathing in six patients with residual mixed apneas at best PAP settings. The authors concluded that low concentrations of CO2 added to conventional PAP can effectively control severe, treatment-resistant, mixed obstructive, and central sleep-disordered breathing. However, CO2 supplementation is still used in experimental research due to concerns about the safety and side effects of CO2. Regardless, CO2 inhalation therapy is still considered a possible alternative approach for the treatment of CSA.[84]
Future Directions
Despite so many findings from several studies, many questions regarding TECSA remain. First, the reported prevalence and natural course of TECSA vary widely in different studies. Therefore, it is necessary to carry out a large epidemiological survey with rigorous and superior scientific design. Second, the clinical features and risk factors for this disease are not specific. Therefore, identifying TECSA patients with OSA and/or CSA and what clinical information is most important and useful in predicting the development and outcomes of TECSA is of paramount importance. To clarify the pertinent aspects of TECSA, evaluating the baseline demographic and polysomnographic data of TECSA patients more carefully and comprehensively is essential. Third, novel viable treatment approaches are needed, and the possible development of an optimal treatment strategy based on clinical information should be investigated as well. Future studies that include basic scientific research and clinical trials on TECSA are also required.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81670084, No. 81600067, and No.81970084).
Conflicts of interest
None.
Footnotes
How to cite this article: Zhang J, Wang L, Guo HJ, Wang Y, Cao J, Chen BY. Treatment-emergent central sleep apnea: a unique sleep-disordered breathing. Chin Med J 2020;133:2721–2730. doi: 10.1097/CM9.0000000000001125
References
- 1.American Academy of Sleep Medicine International Classification of Sleep Disorders. American Academy of Sleep Medicine, 3rd ed.Darien, IL, USA:2014. [Google Scholar]
- 2.Liu D, Armitstead J, Benjafield A, Shao S, Malhotra A, Cistulli PA, et al. Trajectories of emergent central sleep apnea during CPAP therapy. Chest 2017; 152:751–760. doi: 10.1016/j.chest.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nigam G, Pathak C, Riaz M. A systematic review on prevalence and risk factors associated with treatment-emergent central sleep apnea. Ann Thorac Med 2016; 11:202–210. doi: 10.4103/1817-1737.185761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohan A, Henderson J, Mador MJ. Mandibular advancement device-emergent central sleep apnea can resolve spontaneously: a case report. J Clin Sleep Med 2016; 12:137–138. doi: 10.5664/jcsm.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avidan AY, Guilleminault C, Robinson A. The development of central sleep apnea with an oral appliance. Sleep Med 2016; 7:187–191. doi: 10.1016/j.sleep.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Kuzniar TJ, Kovacevic-Ristanovic R, Freedom T. Complex sleep apnea unmasked by the use of a mandibular advancement device. Sleep Breath 2011; 15:249–252. doi: 10.1007/s11325-010-0459-8. [DOI] [PubMed] [Google Scholar]
- 7.Gindre L, Gagnadoux F, Meslier N, Fleury B, Gustin JM, Racineux JL. Central apnea developing during treatment with a mandibular advancement device (in French). Rev Mal Respir 2006; 23:477–480. doi: 10.1019/20064167. [DOI] [PubMed] [Google Scholar]
- 8.Corcoran S, Mysliwiec V, Niven AS, Fallah D. Development of central sleep apnea after maxillofacial surgery for obstructive sleep apnea. J Clin Sleep Med 2009; 5:151–153. doi: 10.5664/jcsm.27444. [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein C, Kuzniar TJ. The emergence of central sleep apnea after surgical relief of nasal obstruction in obstructive sleep apnea. J Clin Sleep Med 2012; 8:321–322. doi: 10.5664/jcsm.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nigam G, Riaz M, Chang ET, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med 2018; 13:86–91. doi: 10.4103/atm.ATM_321_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pépin JL, Woehrle H, Liu D, Shao S, Armitstead JP, Cistulli PA, et al. Adherence to positive airway therapy after switching from CPAP to ASV: a big data analysis. J Clin Sleep Med 2018; 14:57–63. doi: 10.5664/jcsm.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilmartin GS, Daly RW, Thomas RJ. Recognition and management of complex sleep-disordered breathing. Curr Opin Pulm Med 2015; 11:485–493. doi: 10.1097/01.mcp.0000183061.98665.b0. [DOI] [PubMed] [Google Scholar]
- 13.Morgenthaler TI, Kagramanov V, Hanak V, Decker PA. Complex sleep apnea syndrome: is it a unique clinical syndrome? Sleep 2006; 29:1203–1209. doi: 10.1093/sleep/29.9.1203. [DOI] [PubMed] [Google Scholar]
- 14.Guilleminault C, Cummiskey J. Progressive improvement of apnea index and ventilatory response to CO2 after tracheostomy in obstructive sleep apnea syndrome. Am Rev Respir Dis 1982; 126:14–20. doi: 10.1164/arrd.1982.126.1.14. [DOI] [PubMed] [Google Scholar]
- 15.Fay MB, Goodday R. The emergence of central sleep apnea after maxillo-mandibular advancement surgery for obstructive sleep apnea. J Oral Maxillofac Surg 2019; 77:2303–2307. doi: 10.1016/j.joms.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Westhoff M, Arzt M, Litterst P. Prevalence and treatment of central sleep apnoea emerging after initiation of continuous positive airway pressure in patients with obstructive sleep apnoea without evidence of heart failure. Sleep Breath 2012; 16:71–78. doi: 10.1007/s11325-011-0486-0. [DOI] [PubMed] [Google Scholar]
- 17.Verbraecken J. Complex sleep apnoea syndrome. Breathe 2013; 9:373–380. doi: 10.1183/20734735.042412. [Google Scholar]
- 18.Javaheri S, Smith J, Chung E. The prevalence and natural history of complex sleep apnea. J Clin Sleep Med 2009; 5:205–211. doi: 10.1007/s11069-009-9396-x. [PMC free article] [PubMed] [Google Scholar]
- 19.Kuzniar TJ, Pusalavidyasagar S, Gay PC, Morgenthaler TI. Natural course of complex sleep apnea: a retrospective study. Sleep Breath 2008; 12:135–139. doi: 10.1007/s11325-007-0140-z. [DOI] [PubMed] [Google Scholar]
- 20.Endo Y, Suzuki M, Inoue Y, Sato M, Namba K, Hasegawa M, et al. Prevalence of complex sleep apnea among Japanese patients with sleep apnea syndrome. Tohoku J Exp Med 2008; 215:349–354. doi: 10.1620/tjem.215.349. [DOI] [PubMed] [Google Scholar]
- 21.Cassel W, Canisius S, Becker HF, Leistner S, Ploch T, Jerrentrup A, et al. A prospective polysomnographic study on the evolution of complex sleep apnoea. Eur Respir J 2011; 38:329–337. doi: 10.1183/09031936.00162009. [DOI] [PubMed] [Google Scholar]
- 22.Khan MT, Franco RA. Complex sleep apnea syndrome. Sleep Disord 2014; 2014:798487.doi: 10.1155/2014/798487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neu D, Balkissou AD, Mairesse O, Pefura-Yone EW, Noseda A. Complex sleep apnea at auto-titrating CPAP initiation: prevalence, significance and predictive factors. Clin Respir J 2017; 11:200–209. doi: 10.1111/crj.12325. [DOI] [PubMed] [Google Scholar]
- 24.Bitter T, Westerheide N, Hossain MS, Lehmann R, Prinz C, Kleemeyer A, et al. Complex sleep apnoea in congestive heart failure. Thorax 2011; 66:402–407. doi: 10.1136/thx.2010.146522. [DOI] [PubMed] [Google Scholar]
- 25.Bazurto Zapata MA, Martinez-Guzman W, Vargas-Ramirez L, Herrera K, Gonzalez-Garcia M. Prevalence of central sleep apnea during continuous positive airway pressure (CPAP) titration in subjects with obstructive sleep apnea syndrome at an altitude of 2640 m. Sleep Med 2015; 16:343–346. doi: 10.1016/j.sleep.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 26.Pagel JF, Kwiatkowski C, Parnes B. The effects of altitude associated central apnea on the diagnosis and treatment of obstructive sleep apnea: comparative data from three different altitude locations in the Mountain West. J Clin Sleep Med 2011; 7:610–615. doi: 10.5664/jcsm.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miao ZB, Wang YY, Sun R, Li HL, Huang DJ, Huang LH, et al. Incidence and influencing factors of treatment emergent central sleep apnea in patients with OSAS at high altitude (in Chinese). Nati Med J China 2017; 99:1864–1869. doi: 10.3760/cma.j.issn.0376-2491.2017.12.008. [Google Scholar]
- 28.Muza RT. Central sleep apnoea-a clinical review. J Thorac Dis 2015; 7:930–937. doi: 10.3978/j.issn.2072-1439.2015.04.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malhotra A, Owens RL. What is central sleep apnea? Respir Care 2010; 55:1168–1178. [PMC free article] [PubMed] [Google Scholar]
- 30.Younes M, Ostrowski M, Atkar R, Laprairie J, Siemens A, Hanly P. Mechanisms of breathing instability in patients with obstructive sleep apnea. J Appl Physiol 2007; 103:1929–1941. doi: 10.1152/japplphysiol.00561.2007. [DOI] [PubMed] [Google Scholar]
- 31.Johnson KG, Johnson DC. Bilevel positive airway pressure worsens central apneas during sleep. Chest 2005; 128:2141–2150. doi: 10.1378/chest.128.4.2141. [DOI] [PubMed] [Google Scholar]
- 32.Chowdhuri S, Shanidze I, Pierchala L, Belen D, Mateika JH, Badr MS. Effect of episodic hypoxia on the susceptibility to hypocapnic central apnea during NREM sleep. J Appl Physiol 2010; 108:369–377. doi: 10.1152/japplphysiol.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. Sleep 1997; 20:654–675. doi: 10.1093/sleep/20.8.654. [DOI] [PubMed] [Google Scholar]
- 34.Lombardi C, Caravita S, Parati G. Central sleep apnea during continuous positive airway pressure therapy in obstructive sleep apnea patients: from the compliance to adaptation, maladaptation and reflexes. J Thorac Dis 2017; 9:4152–4156. doi: 10.21037/jtd.2017.09.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dernaika T, Tawk M, Nazir S, Younis W, Kinasewitz GT. The significance and outcome of continuous positive airway pressure related central sleep apnea during split-night sleep studies. Chest 2007; 132:81–87. doi: 10.1378/chest.06-2562. [DOI] [PubMed] [Google Scholar]
- 36.Tkacova R, Niroumand M, Lorenzi-Filho G, Bradley TD. Overnight shift from obstructive to central apneas in patients with heart failure: role of PCO2 and circulatory delay. Circulation 2001; 103:238–243. doi: 10.1161/01.CIR.103.2.238. [DOI] [PubMed] [Google Scholar]
- 37.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2001; 163:1181–1190. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]
- 38.Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest 2007; 131:595–607. doi: 10.1378/chest.06.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanchina M, Robinson K, Corrao W, Donat W, Sands S, Malhotra A. Clinical use of loop gain measures to determine continuous positive airway pressure efficacy in patients with complex sleep apnea: a pilot study. Ann Am Thorac Soc 2015; 12:1351–1357. doi: 10.1513/AnnalsATS.201410-469BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carberry JC, Amatoury J, Eckert DJ. Personalized management approach for OSA. Chest 2018; 153:744–755. doi: 10.1016/j.chest.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Malhotra A, Bertisch S, Wellman A. Complex sleep apnea: it isn’t really a disease. J Clin Sleep Med 2008; 4:406–408. doi: 10.5664/jcsm.27273. [PMC free article] [PubMed] [Google Scholar]
- 42.Horner RL, Rivera MP, Kozar LF, Phillipson EA. The ventilatory response to arousal from sleep is not fully explained by differences in CO2 levels between sleep and wakefulness. J Physiol 2001; 534:881–890. doi: 10.1111/j.1469-7793.2001.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khoo MC, Koh SS, Shin JJ, Westbrook PR, Berry RB. Ventilatory dynamics during transient arousal from NREM sleep: implications for respiratory control stability. J Appl Physiol 1996; 80:1475–1484. doi: 10.1152/jappl.1996.80.5.1475. [DOI] [PubMed] [Google Scholar]
- 44.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med 2004; 169:623–633. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 45.Jordan AS, McEvoy RD, Edwards JK, Schory K, Yang CK, Catcheside PG, et al. The influence of gender and upper airway resistance on the ventilatory response to arousal in obstructive sleep apnoea in humans. J Physiol 2004; 558:993–1004. doi: 10.1113/jphysiol.2004.064238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med 2005; 172:1363–1370. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- 47.Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 2017; 69:841–858. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol 2013; 3:141–163. doi: 10.1002/cphy.c110057. [DOI] [PubMed] [Google Scholar]
- 49.Salloum A, Rowley JA, Mateika JH, et al. Increased propensity for central apnea in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure. Am J Respir Crit Care Med 2010; 181:189–193. doi: 10.1164/rccm.200810-1658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khoo MC, Gottschalk A, Pack AI. Sleep-induced periodic breathing and apnea: a theoretical study. J Appl Physiol 1991; 70:2014–2024. doi: 10.1152/jappl.1991.70.5.2014. [DOI] [PubMed] [Google Scholar]
- 51.Hall MJ, Xie A, Rutherford R, Ando S, Floras JS, Bradley TD. Cycle length of periodic breathing in patients with and without heart failure. Am J Respir Crit Care Med 1996; 154:376–381. doi: 10.1164/ajrccm.154.2.8756809. [DOI] [PubMed] [Google Scholar]
- 52.Pusalavidyasagar SS, Olson EJ, Gay PC, Morgenthaler TI. Treatment of complex sleep apnea syndrome: a retrospective comparative review. Sleep Med 2006; 7:474–479. doi: 10.1016/j.sleep.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Lehman S, Antic NA, Thompson C, Catcheside PG, Mercer J, McEvoy RD. Central sleep apnea on commencement of continuous positive airway pressure in patients with a primary diagnosis of obstructive sleep apnea-hypopnea. J Clin Sleep Med 2007; 3:462–466. [PMC free article] [PubMed] [Google Scholar]
- 54.Moro M, Gannon K, Lovell K, Merlino M, Mojica J, Bianchi MT. Clinical predictors of central sleep apnea evoked by positive airway pressure titration. Nat Sci Sleep 2016; 8:259–266. doi: 10.2147/NSS.S110032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yaegashi H, Fujimoto K, Abe H, Orii K, Eda S, Kubo K. Characteristics of Japanese patients with complex sleep apnea syndrome: a retrospective comparison with obstructive sleep apnea syndrome. Intern Med 2009; 48:427–432. doi: 10.2169/internalmedicine.48.1459. [DOI] [PubMed] [Google Scholar]
- 56.Montesi SB, Bakker JP, Macdonald M, Hueser L, Pittman S, White DP, et al. Air leak during CPAP titration as a risk factor for central apnea. J Clin Sleep Med 2013; 9:1187–1191. doi: 10.5664/jcsm.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lei F, Tan L, Li TM, Ren R, Zhou JY, Zhou XY, et al. Association between mixed sleep apnea and treatment-emergent central sleep apnea (in Chinese). Nati Med J China 2019; 99:1864–1869. doi: 10.3760/cma.j.issn.0376-2491.2019.24.006. [DOI] [PubMed] [Google Scholar]
- 58.Herkenrath S, Pavsic K, Treml M, Hagmeyer L, Randerath W. Mixed apnea metrics during diagnostic polysomnographies in obstructive sleep apnea patients with/without treatment-emergent central sleep apnea. Eur Respir J 2019; 54: Suppl. 63: A824.doi: 10.1183/13993003.congress-2019.PA824. [Google Scholar]
- 59.Mulgrew AT, Lawati NA, Ayas NT. Residual sleep apnea on polysomnography after 3 months of CPAP therapy: clinical implications, predictors and patterns. Sleep Med 2010; 11:119–125. doi: 10.1016/j.sleep.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 60.Kuźniar TJ, Kasibowska-Kuźniar K, Ray DW, Freedom T. Clinical heterogeneity of patients with complex sleep apnea syndrome. Sleep Breath 2013; 17:1209–1214. doi: 10.1007/s11325-013-0825-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walker JM, Farney RJ, Rhondeau SM, Boyle KM, Valentine K, Cloward TV, et al. Chronic opioid use is a risk factor for the development of central sleep apnea and ataxic breathing. J Clin Sleep Med 2007; 3:455–461. doi: 10.5664/jcsm.26908. [PMC free article] [PubMed] [Google Scholar]
- 62.Javaheri S, Malik A, Smith J, Chung E. Adaptive pressure support servoventilation: a novel treatment for sleep apnea associated with use of opioids. J Clin Sleep Med 2008; 4:305–310. [PMC free article] [PubMed] [Google Scholar]
- 63.Morgenthaler TI. The quest for stability in an unstable world: adaptive servoventilation in opioid induced complex sleep apnea syndrome. J Clin Sleep Med 2008; 4:321–323. doi: 10.5664/jcsm.27231. [PMC free article] [PubMed] [Google Scholar]
- 64.Fahim A, Johnson AO. Chronic opioid use: a risk factor for central sleep apnoea and successful therapy with adaptive pressure support servo-ventilation. J R Coll Physicians Edinb 2012; 42:314–316. doi: 10.4997/JRCPE.2012.407. [DOI] [PubMed] [Google Scholar]
- 65.Orr JE, Malhotra A, Sands SA. Pathogenesis of central and complex sleep apnoea. Respirology 2017; 22:43–52. doi: 10.1111/resp.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Loewen A, Ostrowski M, Laprairie J, Atkar R, Gnitecki J, Hanly P, et al. Determinants of ventilatory instability in obstructive sleep apnea: inherent or acquired? Sleep 2009; 32:1355–1365. doi: 10.1093/sleep/32.10.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Correia S, Martins V, Sousa L, Moita J, Teixeira F, Dos Santos JM. Clinical impact of adaptive servoventilation compared to other ventilatory modes in patients with treatment-emergent sleep apnea, central sleep apnea and Cheyne-Stokes respiration. Rev Port Pneumol 2015; 21:132–137. doi: 10.1016/j.rppnen.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 68.Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep 2007; 30:468–475. doi: 10.1093/sleep/30.4.468. [DOI] [PubMed] [Google Scholar]
- 69.Allam JS, Olson EJ, Gay PC, Morgenthaler TI. Efficacy of adaptive servoventilation in treatment of complex and central sleep apneasyndromes. Chest 2007; 132:1839–1346. doi: 10.1378/chest.07-1715. [DOI] [PubMed] [Google Scholar]
- 70.Brown SE, Mosko SS, Davis JA, Pierce RA, Godfrey-Pixton TV. A retrospective case series of adaptive servoventilation for complex sleep apnea. J Clin Sleep Med 2011; 7:187–195. doi: 10.1016/j.jocn.2010.07.104. [PMC free article] [PubMed] [Google Scholar]
- 71.Kuzniar TJ, Patel S, Nierodzik CL, Smith LC. Comparison of two servo ventilator devices in the treatment of complex sleep apnea. Sleep Med 2011; 2:538–541. doi: 10.1016/j.sleep.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 72.Randerath WJ, Galetke W, Kenter M, Richter K, Schafer T. Combined adaptive servo-ventilation and automatic positive airway pressure (anticyclic modulated ventilation) in co-existing obstructive and central sleep apnea syndrome and periodic breathing. Sleep Med 2009; 10:898–903. doi: 10.1016/j.sleep.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 73.Dellweg D, Kerl J, Hoehn E, Wenzel M, Koehler D. Randomized controlled trial of noninvasive positive pressure ventilation (NPPV) versus servo ventilation in patients with CPAP-induced central sleep apnea (complex sleep apnea). Sleep 2013; 36:1163–1171. doi: 10.5665/sleep.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morgenthaler TI, Kuzniar TJ, Wolfe LF, Willes L, McLain WC, 3rd, Goldberg R. The complex sleep apnea resolution study: a prospective randomized controlled trial of continuous positive airway pressure versus adaptive servoventilation therapy. Sleep 2014; 37:927–934. doi: 10.5665/sleep.3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuzniar TJ, Morgenthaler TI. Treatment of complex sleep apnea syndrome. Curr Treat Options Neurol 2008; 10:336–341. doi: 10.1007/s11940-008-0036-7. [DOI] [PubMed] [Google Scholar]
- 76.Glidewell RN, Orr WC, Imes N. Acetazolamide as an adjunct to CPAP treatment: a case of complex sleep apnea in a patient on long-acting opioid therapy. J Clin Sleep Med 2009; 5:63–64. doi: 10.5664/jcsm.27394. [PMC free article] [PubMed] [Google Scholar]
- 77.Eckert DJ, Malhotra A, Wellman A, White DP. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep 2014; 37:811–819. doi: 10.5665/sleep.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eckert DJ, Owens RL, Kehlmann GB, Wellman A, Rahangdale S, Yim-Yeh S, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci 2011; 120:505–514. doi: 10.1042/CS20100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol 2008; 162:144–151. doi: 10.1016/j.resp.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Javaheri S, Ahmed M, Parker TJ, Brown CR. Effects of nasal O2 on sleep related disordered breathing in ambulatory patients with stable heart failure. Sleep 1999; 22:1101–1106. doi: 10.1093/sleep/22.8.1101. [DOI] [PubMed] [Google Scholar]
- 81.Andreas S, Clemens C, Sandholzer H, Figulla HR, Kreuzer H. Improvement of exercise capacity with treatment of Cheyne–Stokes respiration in patients with congestive heart failure. J Am College Cardiol 1996; 27:1486–1490. doi: 10.1016/0735-1097(96)00024-1. [DOI] [PubMed] [Google Scholar]
- 82.Kuźniar TJ, Morgenthaler TI. Treatment of complex sleep apnea syndrome. Chest 2012; 142:1049–1057. doi: 10.1378/chest.11-3223. [DOI] [PubMed] [Google Scholar]
- 83.Thomas RJ, Daly RW, Weiss JW. Low-concentration carbon dioxide is an effective adjunct to positive airway pressure in the treatment of refractory mixed central and obstructive sleep-disordered breathing. Sleep 2005; 28:69–77. doi: 10.1093/sleep/28.1.69. [DOI] [PubMed] [Google Scholar]
- 84.Thomas RJ. Alternative approaches to treatment of central sleep apnea. Sleep Med Clin 2014; 9:87–104. doi: 10.1016/j.jsmc.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


