Abstract
There is no definitive neural marker of suicidal thoughts and behaviors (STB) or non-suicidal self-injury (NSSI), and relative to adults, research in youth is more limited. This comprehensive review focuses on magnetic resonance imaging (MRI) studies reporting structural and functional neural correlates of STB and NSSI in youth to: (i) elucidate shared and independent neural alternations, (ii) clarify how developmental processes may interact with neural alterations to confer risk, and (iii) provide recommendations based on convergence across studies. Forty-seven articles were reviewed (STB = 27; NSSI = 20), and notably, 63% of STB articles and 45% of NSSI articles were published in the previous 3 years. Structural MRI research suggests reduced volume in the ventral prefrontal and orbitofrontal cortices among youth reporting STB, and there is reduced anterior cingulate cortex volume related to STB and NSSI. With regard to functional alterations, blunted striatal activation may characterize STB and NSSI youth, and there is reduced frontolimbic task-based connectivity in suicide ideators and attempters. Resting state functional connectivity findings highlight reduced positive connectivity between the default mode network and salience network in attempters, and self-injurers exhibit frontolimbic alterations. Together, suicidal and non-suicidal behaviors are related to top-down and bottom-up neural alterations, which may compromise approach, avoidance, and regulatory systems. Future longitudinal research with larger and well-characterized samples, especially those integrating ambulatory stress assessments, will be well-positioned to identify novel targets that may improve early identification and treatment for youth with STB and NSSI.
Keywords: Suicide Ideation, Suicide Attempt, NSSI, Neuroimaging, Neurodevelopmental, Neuromaturation
Suicidal thoughts and behaviors (STB) and non-suicidal self-injury (NSSI) in youth are major public health concerns. Although STB and NSSI often co-occur (1), their etiologies are complex and only partially overlap, suggesting both shared and unique vulnerability factors that potentiate STB and NSSI (2, 3). Recent data show that 12.1% of adolescents think about suicide and 4.1% have made an attempt (4). This is concerning, as upwards of 70% of completers are first time attempters, with suicide deaths markedly higher among males (e.g., 5). By comparison, 15–20% of adolescents report lifetime NSSI engagement (6), disproportionately affecting females (7). These sex differences are important to consider, particularly as NSSI is often a precursor to suicidal behaviors in females, but many males attempting suicide report little or no NSSI history (8, 9). Critically, the motivation for engaging in suicidal (e.g., intolerance of psychological pain (10); perceived burdensomeness (11)) versus non-suicidal behaviors (e.g., self-punishment (12); social signaling (13)) also may differ, perhaps influencing why NSSI often desists in adulthood (14), whereas STB may persist (15, 16). Taken together, contemporary models underscore the importance of elucidating interactive, and potentially transactional, processes that may differentially shape the trajectories of STB and NSSI during the transition from adolescence to adulthood and accordingly, focus on identifying biological (e.g., neural correlates) and environmental (e.g., child abuse) factors in the STB and NSSI pathways (2, 3, 13, 17–20).
The diathesis-stress model provides a framework to understand vulnerability to self-injurious behaviors. Neural alterations are biological diatheses that may be a necessary but not sufficient predisposition to engage in self-injurious behaviors. Coupled with specific environmental exposures—particularly acute stress (e.g., peer victimization, interpersonal loss)—the likelihood of suicidal (21–23) and non-suicidal (24–26) behaviors may increase. Childhood and adolescence represent a unique developmental phase to unpack neural diatheses and stressors that may be separable from adults. From a neurodevelopmental perspective, this is a period characterized by asynchronicity between limbic and prefrontal systems (27). Although normative, this has a direct effect on motivated behavior, particularly as it relates to the neural circuitry of approach, avoidance, and regulatory systems. Within the triadic model of motivated behavior, the approach system is guided by the ventral striatum, the avoidance system is largely controlled by the amygdala, and the regulatory system—generally managed by the prefrontal cortex—is believed to balance approach and avoidance behaviors (28). Indeed, Casey and colleagues have long advanced that risky behaviors in youth stem from earlier maturation of subcortical regions relative to immature prefrontal systems (29). By contrast, STB and NSSI may manifest not when there is asynchronicity between limbic and prefrontal systems, but perhaps, due to deficiencies at each level—across approach, avoidance, and regulatory systems. Although failure with any one system may result in the emergence of debilitating symptoms or risky behaviors (e.g., (30, 31)), when each system is compromised, it undermines the capacity to engage in compensatory strategies (e.g., future-oriented thinking, cognitive flexibility) that would otherwise limit the tendency to engage in suicidal and non-suicidal behaviors. This is consistent with a recent model summarizing extant STB findings (32), showing that alterations within the extended ventral prefrontal cortex (VPFC) system may potentiate suicidal thinking given prominent roles in negative self-referential thinking and rumination (33, 34). Suicidal thinking may then be exacerbated by dorsolateral prefrontal cortex (DLPFC), inferior frontal gyrus (IFG), rostral prefrontal cortex (PFC), and dorsal anterior cingulate cortex (dACC) alterations, as this directly bears on effective cognitive control, flexibility, and decision-making (35). Perturbations in bottom-up and top-down connections between these extended systems may, for some, facilitate the transition from suicidal ideation to behavior. It remains unknown, however, whether this relates to STB in youth, and if this model is applicable to NSSI.
Neural alterations do not operate in isolation. Rather, they interact dynamically with stress (2, 3, 36, 37), a point underscored by the fact that stress is prominently featured in many leading STB (11, 20, 38) and NSSI (25, 39) theories. Childhood and adolescence reflect a unique developmental period whereby there is a progressive shift towards autonomy from parents and greater reliance on peers (40). Interpersonal stressors occur in great abundance during this life stage (23)—a byproduct of navigating puberty (41), fostering close friendships (42), exploring romantic relationships (43), and increasingly, using social media (44). Acute interpersonal stress may serve as proximal triggers that interact with neural diatheses, which we maintain in this review, may facilitate the transition from ideation to action—for both suicidal and non-suicidal behaviors.
Presently, there are no definitive neural markers of STB or NSSI. Several prior reviews have highlighted brain regions implicated in suicidal (32, 45–49) and non-suicidal behaviors (39, 50). This review focuses on extant magnetic resonance imaging (MRI) studies reporting structural and functional neural correlates of STB and NSSI in youth with the aim of: (i) elucidating shared and unique neural alternations, (ii) clarifying how developmental processes may interact with neural alterations to confer risk, and (iii) providing recommendations based on convergence across studies.
Method
A PubMed and Google Scholar literature search through January 15, 2020 was conducted to identify original research using the search terms: “MRI,” “magnetic resonance imaging,” “structural MRI,” “fMRI,” “functional MRI,” “functional magnetic resonance imaging,” “functional connectivity,” “resting state fMRI,” “resting state connectivity” in combination with “suicide,” “suicide attempt,” “suicidal ideation,” “suicidal behavior,” “non-suicidal self-injury,” “parasuicidal behaviors,” and “self-harm.” Articles were selected if they: (i) were published in an English-language peer-reviewed journal, (ii) included participants under age 26, (iii) included participants with lifetime STB and/or NSSI, and (iv) focused on structural, functional, or resting state MRI. There were no inclusion requirements based on STB or NSSI assessments (Tables S1–S3 for clinical measures) or MRI scanners. Several reviewed publications assess neural correlates of STB among youth with lifetime NSSI, and conversely, NSSI studies include STB youth (Tables S1–S3 for sample overlap). Extant research is summarized, but a pooled meta-analytic estimate of effects is not provided.
Results
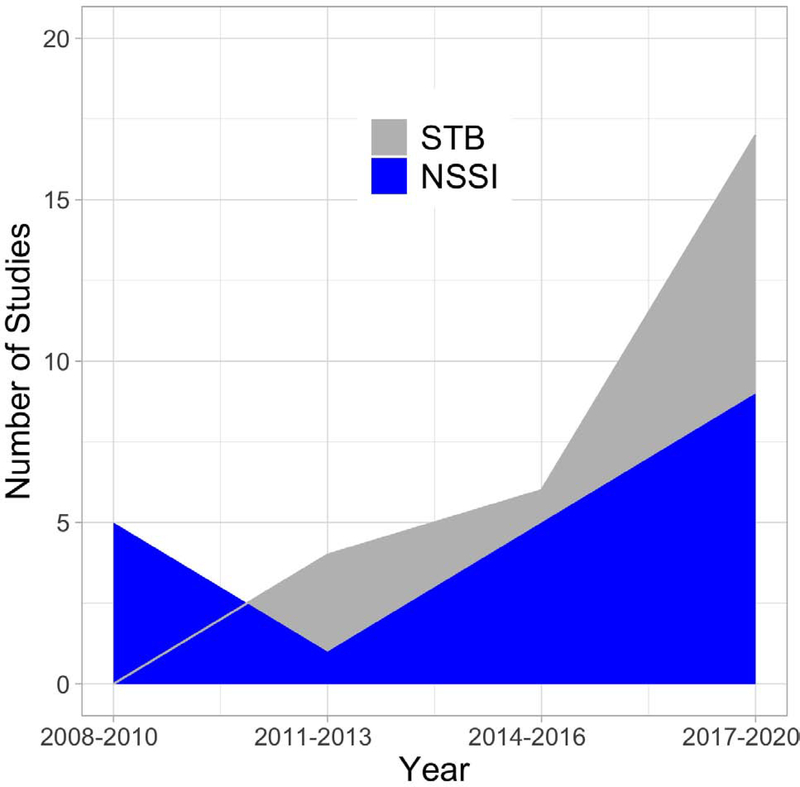
Forty-seven articles satisfy inclusion criteria (STB = 27; NSSI = 20; Figure 1). The majority of participants in these studies reported lifetime major depressive disorder (MDD), bipolar disorder (BD), and/or borderline personality disorder (BPD) (Table 1–3 for sample characteristics). There is enormous heterogeneity in methodological approaches. Most STB studies compare suicide attempters versus a psychiatric or healthy comparison group. Few studies compare attempters versus ideators. NSSI samples are typically compared with either psychiatric or healthy youth, with some research testing correlates among NSSI youth only. The vast majority of research is cross-sectional.
Figure 1.
Suicidal thoughts and behaviors (STB; gray) and non-suicidal self-injury (NSSI; blue) neuroimaging studies included in this comprehensive review. Publications are divided into 3-year bins and calculated separately for STB and NSSI.
Table 1.
Structural alterations associated with suicidal thoughts and behaviors and non-suicidal self-injury
| Publication | Sample | Age | Method | Select Findings |
|---|---|---|---|---|
| Suicidal Thoughts and Behaviors | ||||
| Fan et al., 2019 (54) | BD+SA = 21 BD = 25 MDD+SA = 19 MDD = 18 |
14–25 | Whole Brain, SPM8, GMV | 1,4BD+SA, MDD+SA > BD, MDD ↓ Left VPFC (BA11); MDD+SA > MDD, BD+SA, BD ↑ Left VPFC (BA47) |
| Fradkin et al., 2017 (57) | MDD+SA = 29 HC = 29 |
12–19 | Whole Brain, FreeSurfer/Qdec, Cortical Thickness, Surface Area | 1,3MDD+SA ↓ VMPFC cortical thickness ↑ Motor impulsivity; MDD+SA ↑ Right paracentral lobule cortical thickness ↑ Non-planning impulsivity |
| Goodman et al., 2011 (59) | BPD+MDD = 13 HC = 13 |
13–17 | ROI (Cingulate, PFC), GMV/WMV | 1,3↑ SA ↓ BA24 volume, ↑ BA23 WMV |
| Ho et al., 2018 (58) | §Community Sample = 152 | 9–13 | ROI (Caudate, NAcc, Putamen), FreeSurfer, GMV | 1,3↓ Bilateral Putamen predicts ↑ Implicit SI |
| Huber et al., 2019 (55) | BD+SA = 15 BD = 18 HC = 25 |
13–21 | ROI (OFC), FreeSurfer, Thickness/Volume | 1,3BD+SA > HC ↓ Left OFC volume, ↓ Bilateral OFC thickness; BD+SA > BD ↓ Right OFC thickness; ↑ SA lethality ↓ Bilateral OFC volume; ↑ SI ↓ left OFC volume |
| Johnston et al., 2017a (53) | BD+SA = 26 BD = 42 HC = 45 |
14–25 | Whole Brain, SPM5, GMV | 1,4 BD+SA > BD ↓ Right OFC, Right Hippocampus, Bilateral Cerebellum GMV; BD+SA > HC ↓ Right Hippocampus GMV |
| Lippard et al., 2019 (56) | Future SA = 17 No Future SA = 29 |
13–25 | Whole Brain, SPM12, GMV | 1,3Future SA > No Future SA↓ VPFC GMV (Left BA11, Right Ventral BA10, Right BA47), ↓ Left DRPFC (BA10) |
| McLellan et al., 2018 (60) | MDD+SA = 14 MDD = 14 HC = 17 |
12–21 | ROI (rSTG, Frontal, Temporal Regions), FreeSurfer, Cortical Thickness/Volume | 1,3MDD+SA > HC ↓ rSTG volume |
| Pan et al., 2015 (61) | MDD+SA = 28 MDD = 31 HC = 41 |
12–17 | Whole Brain, FreeSurfer/Qdec, Cortical Thickness/ GMV/WMV | 1,3MDD+SA > HC ↓ rSTG GMV |
| Non-Suicidal Self-Injury | ||||
| Ando et al., 2018 (64) |
§NSSI+SA = 16 §NSSI = 13 HC = 21 |
*^NSSI = 15.9±1.3 *HC = 15.8±1.1 |
ROI (PFC, ACC, Insula, Thalamus, Hippocampus, Amygdala), FreeSurfer, GMV | 1,5All NSSI > HC ↓ Insula GMV, ↓ ACC GMV; NSSI+SA> NSSI, HC ↓ ACC GMV |
| Beauchaine et al., 2019 (62) |
§NSSI = 20 §No NSSI = 20 |
13–19 | Whole Brain, SPM12, GMV | 1,5NSSI > No NSSI ↓ Bilateral Insular GMV, ↓ Right IFG |
| Chanen et al., 2008c (66) | BPD = 20 HC = 20 |
*BPD = 17.3±1.1 *HC = 19.0±2.2 |
ROI (OFC, Amygdala, Hippocampus), Hand Tracing, GMV |
1,4 No association between OFC GMV and number of episodes; 1,5 No association between Amyg or Hippocampal GMV and number of episodes Sex: BPD Males > BPD Females, HC ↓ Amyg GMV |
| Takahashi et al., 2009b (65) | BPD = 20 HC = 20 |
*BPD = 17.3±1.1 *HC = 19.0±2.2 |
ROI (Insula), Hand Tracing, GMV | 2,3No association between Insula GMV and number of episodes; Null findings between BPD with and without episodes in the past 6 months |
| Takahashi et al., 2009b (67) | BPD = 20 HC = 20 |
*BPD = 17.3±1.1 *HC = 19.0±2.2 |
ROI (AI, CSP), Hand Tracing, Length | 1,4No association between AI or CSP length and number of episodes |
| Takahashi et al., 2010b (68) | BPD = 20 HC = 20 |
*BPD = 17.3±1.1 *HC = 19.0±2.2 |
ROI (STG), Hand Tracing, Volume | 1,3No association between STG volume and number of episodes; Null findings between BPD with and without episodes in the past 6 months |
| Whittle et al., 2009c (69) | BPD = 15 HC = 15 |
*BPD = 17.4±1.15 *HC = 19.7±2.18 |
ROI (ACC), Hand Tracing, GMV | 1,5↓ Left ACC GMV ↑ Number of episodes |
Note. Bold = Strong methodological approach (i.e., clinical-control comparison, interview assessment of STB/NSSI, data analysis)
Age range not reported
Age range for all NSSI participants including the NSSI+SA group
Also includes fMRI (Table 2)
Same sample
Comorbid disorders see Table S1 for inclusion and exclusion criteria
Age effects not reported
Age effects null
Sex effects not reported
Sex effects null
Female only sample Group.
BD = Bipolar Disorder; BPD = Borderline Personality Disorder; Future SA = Participant made a suicide attempt between baseline and follow-up; HC = Healthy Control; MDD = Major Depressive Disorder; No Future SA = Participant did not make a suicide attempt between baseline and follow-up; NSSI = Non-Suicidal Self-Injury; SA = Suicide Attempt
Method. GMV = Gray Matter Volume; ROI = Region of Interest; WMV = White Matter Volume
Region. ACC = Anterior Cingulate Cortex; AI = Adhesio Interthalamica; Amyg = Amygdala; BA = Brodmann Area; CSP = Cavum Septum Pellucidum; DRPFC = Dorsal Rostral Prefrontal Cortex; IFG = Inferior Frontal Gyrus; NAcc = Nucleus Accumbens; OFC = Orbitofrontal Cortex; PFC = Prefrontal Cortex; rSTG = Right Superior Temporal Gyrus; VMPFC = Ventral Medial Prefrontal Cortex; VPFC = Ventral Prefrontal Cortex
Outcome. SI = Suicidal Ideation
Table 3.
Resting state functional connectivity patterns related to suicidal thoughts and behaviors and non-suicidal self-injury
| Publication | Sample | Age | Method | Select Findings |
|---|---|---|---|---|
| Suicidal Thoughts and Behaviors | ||||
| Cao et al., 2015 (106) | SA = 19 HC = 20 |
*SA: 19.84 ± 1.61 *HC: 20.30 ± 1.72 |
Voxel-wise ReHo | 2,4SA > HC ↑ MFG, Parietal, Precuneus ↓ Cerebellum, Fusiform, Hippocampus, Paraphippocampal, Angular, IFG, MFG |
| Cao et al., 2016a (107) | MDD+SA = 35 MDD = 18 HC = 47 |
15–25 | Voxel-wise ALFF | 2,4MDD+SA > MDD, HC ↑ MTG, STG, MOG |
| Cao et al., 2020a (103) | MDD+SA = 35 MDD = 18 HC = 47 |
15–25 | ICA Networks | 2,4MDD+SA > MDD ↓ aDMN-SN ↑ FPN-SN, FPN-aDMN; 2,4MDD+SA > HC ↓ aDMN-pDMN, aDMN-FPN ↑ FPN-SN |
| Cullen et al., 2014b (100) | MDD = 41 HC = 29 |
12–19 | Seed-Based | 2,4MDD-related AMYG seed did not relate to suicide severity |
| Ordaz et al., 2018c (104) | MDD = 40 | 14–17 | ICA Networks | 1,3↑ Lifetime ideation severity ↓ Coherence in ECN, aDMN, SN |
| Schreiner et al., 2019b (101) | MDD = 58 | 12–19 | Seed-Based | 1,3↑ Suicide severity ↑ Precuneus-Cerebellum, IFG; ↓ PCC-Cerebellum, Cingulate |
| Schwartz et al., 2019c (105) | MDD = 33 | 14–17 | ICA Networks | 1,36M Follow-Up: ↓ Ideation severity ↑ SN coherence |
| Zhang et al., 2016a (102) | MDD+SA = 35 MDD = 18 HC = 47 |
15–25 | ICA Networks | 1,3MDD+SA > MDD ↑ DMN-Cerebellum, Lingual ↓ DMN-Precuneus |
| Non-Suicidal Self-Injury | ||||
| Cullen et al., 2020d (108) | §NSSI = 18 | 13–21 | Seed-Based | 1,5↓NSSI with NAC treatment = ↓ AMYG-SMA, ↑ AMYG-Inferior Frontal, ↓ NAcc-Superior Medial Frontal |
| Santamarina-Perez et al., 2019 (109) |
§NSSI = 24 HC = 16 |
12–17 | Seed-Based | 1,3NSSI > HC ↓ AMYG-ACC, Insula; ↓mPFC-Precentral/Postcentral Gyri, Insula; ↓NSSI with treatment = ↓ AMYG-ACC, ↑ AMYG-Parahippocampal, ↓ mPFC-AMYG/Striatum, |
| Schreiner et al., 2017d,e (96) |
§NSSI = 25 HC = 20 |
13–21 | Seed-Based | 1,5NSSI > HC ↓ AMYG-ACC/SMA, ↑ AMYG-Angular/Temporal Gyri |
Note. Bold = Strong methodological approach (i.e., clinical-control comparison, interview assessments of STB/NSSI, data analysis, and/or longitudinal design)
Age range not reported
Same sample
Overlapping sample
Also includes fMRI (Table 2)
Age effects not reported
Age effects null
Sex effects not reported
Sex effects null
Female only sample
Comorbid disorders see Table S3 for inclusion and exclusion criteria
Group. HC = Healthy Control; MDD = Major Depressive Disorder; NSSI = Non-Suicidal Self-Injury; SA = Suicide Attempt History
Method. ALFF = Amplitude of Low-Frequency Fluctuation; ReHo = Regional Homogeneity; ICA = Independent Component Analysis
Region. ACC = Anterior Cingulate Cortex; AMYG = Amygdala; IFG = Inferior Frontal Gyrus; MFG = Middle Frontal Gyrus; MOG = Middle Occipital Gyrus; mPFC = Medial Prefrontal Cortex; MTG = Middle Temporal Gyrus; NAcc = Nucleus Accumbens; PCC = Posterior Cingulate Cortex; SFG = Superior Frontal Gyrus; SMA = Supramarginal Gyrus; STG = Superior Temporal Gyrus
Network. aDMN = Anterior DMN; DMN = Default Mode Network; ECN = Executive Control Network; FPN = Fronto-Parietal Network; pDMN = Posterior DMN; SN = Salience Network
Medication. NAC = N-Acetylcysteine
Structural Alterations
STB and NSSI research probing structural differences employ a wide range of methodologies, including region-based and whole-brain (voxel- or vertex-wise) assessments of structural properties. Several studies rely on hand tracing, though the majority use automated methods (e.g., FreeSurfer) to quantify subcortical volumes as well as cortical thickness and surface area. Other studies use voxel-based morphometry (VBM) toolboxes (e.g., SPM) to quantify gray matter volume (GMV). Sample characteristics, structural method, and select findings (including age and sex effects) are summarized in Table 1.
Suicidal Thoughts and Behaviors
Structural alterations within the VPFC and orbitofrontal cortex (OFC), which are key to emotion inhibition, decision-making, and self-control (e.g., (51, 52)), are implicated. Among adolescents with MDD and BD (53, 54), suicide attempters show reduced VPFC and OFC GMV relative to MDD and BD non-attempters, respectively. There is evidence of reduced OFC thickness among BD suicide attempters, and reduced OFC volume negatively correlates with suicide ideation severity and attempt lethality (55). In a prospective study, reduced ventral and rostral prefrontal GMV at baseline differentiates adolescents and young adults with mood disorders who later attempt suicide (56). Studies on PFC differences, however, are complicated by inconsistent laterality effects (left (54, 55) versus right (53)). It also is unclear whether results reflect attempts specifically or the underlying mental disorders more broadly. MDD suicide attempters exhibit increased OFC GMV (left BA47) relative to MDD non-attempters as well as BD attempters and non-attempters (54). However, there is a negative correlation between ventromedial PFC thickness and impulsivity among MDD suicide attempters (57).
Outside of the PFC, BD suicide attempters have reduced hippocampal (53, 54) and bilateral cerebellum (53) GMV compared to BD non-attempters. Additionally, putamen volume is prospectively associated with implicit, but not explicit, suicide ideation (58). Research also implicates the cingulate cortex, as the number of suicide attempts in adolescents with comorbid MDD and BPD correlates with smaller averaged GMV and white matter volume (WMV) in the ventral anterior cingulate cortex (ACC), as well as greater WMV in the ventral posterior cingulate cortex (PCC) (59). Yet, it is not clear if this is driven by suicide attempts or overall mental disorder severity. There also is partial evidence linking temporal lobe structure to STB. MDD attempters have reduced right superior temporal gyrus (STG) GMV relative to healthy adolescents but not compared to MDD non-attempters (60, 61).
Non-Suicidal Self-Injury
Fewer studies examine structural alterations in youth exhibiting NSSI, and this work often: (a) assesses youth reporting parasuicidal behaviors—a term no longer commonly used, as it conflates suicidal and non-suicidal self-injury1 and (b) compares NSSI youth to healthy controls (as opposed to psychiatric samples without NSSI).
There is limited work probing prefrontal differences related to NSSI. One study reports reduced GMV in the right IFG among NSSI adolescents compared to non-depressed individuals (62). Several studies examine the insula, which may modulate subjective emotional experience (63). In comparison to healthy controls, self-injurers show reduced insula GMV (62, 64), but this is not substantiated in BPD youth reporting parasuicidal behaviors (65). Other research found no relationship with the OFC (66), midline structures (67), or STG (68).
Several studies point to ACC alterations. Among adolescents with BPD, a greater number of lifetime parasuicidal behaviors in the past 6 months associates with smaller left ACC volume (69), consistent with findings comparing past-year self-injurers and healthy youth (64). Regarding this latter finding, most NSSI participants (55.2%) reported a lifetime suicide attempt, and given prior work linking anterior ACC volume to suicidal behaviors (59), we cannot determine whether effects are specific to NSSI.
Functional Neuroimaging
Across the STB and NSSI literature, investigators use a range of experimental paradigms (Table S4 for task descriptions). Several studies test task connectivity with psychophysiological interaction (PPI), quantifying condition-related differences in timeseries correlation between activity in a seed region-of-interest (ROI) and other brain regions (70, 71)). Sample characteristics, methodological approaches (e.g., whole brain, ROI), and select findings (including age and sex effects) are provided in Table 2.
Table 2.
Functional neural markers related to suicidal thoughts and behaviors and non-suicidal self-injury
| Publication | Sample | Age | Method | Select Findings |
|---|---|---|---|---|
| Suicidal Thoughts and Behaviors | ||||
| Alarcón et al., 2019a (75) | DEP+SA = 24 DEP+High SI = 27 DEP+Low SI = 31 HC = 38 |
11–18 | PPI (Amyg seed) Emotional Self-Other Morph-Query Task Contrast: Self vs. Other for Happy, Neutral, or Sad |
1,3DEP+SA, DEP+High SI > DEP+Low SI ↑ PPI to DLPFC/dACC, DMPFC, Precuneus; DEP+High SI > DEP+SA, DEP+Low SI ↑ PPI to IPL; DEP+SA ↑ Left PPI to ACC; DEP+High SI ↑ Right PPI to ACC |
| Chase et al., 2019 (84) | MDD+SA = 19 MDD = 22 HC = 23 |
*MDD+SA = 15.6±1.5 *MDD = 16.0±1.4 *HC = 14.7±1.8 |
Whole Brain Dynamic Faces Task Contrast: Face vs. Shape for Angry, Fearful, Happy and Sad |
1,3Null findings between MDD+SA and MDD |
| Harms et al., 2019a (82) | 3 Group Analyses: DEP+High SI = 45 DEP+Low SI = 42 HC = 39 |
11–18 | Whole Brain Cyberball Task Contrast: Exclusion or Inclusion vs. Practice |
1,4DEP+High SI (including SA) > DEP+Low SI, HC ↓ Insula, Putamen across conditions; DEP+SA > DEP+High SI, DEP+Low SI ↑ ACC, SFG, MFG across conditions |
| 4 Group Analyses: DEP+SA = 26 DEP+High SI = 28 DEP+Low SI = 33 HC = 39 |
||||
| Johnston et al., 2017d (53) | BD+SA = 26 BD = 42 HC = 45 |
14–25 | Bilateral Amyg seed connectivity Emotional Face Processing Paradigm Contrast: Emotion vs. Fixation for Fearful, Happy, or Neutral |
1,4BD+SA> BD ↓ PPI to Left Ventral PFC connectivity across neutral and happy conditions (fearful did not survive AlphaSim spatial extent thresholding) and ↓ PPI to Right Rostral PFC for neutral emotion; Among BD+SA ↑ SI ↓ Amyg-right rostral PFC and ↑ Attempt Lethality ↓ Amyg-left VPFC |
| Just et al., 2017 (86) | SI = 17 HC = 17 |
*SI = 22.9±3.6 *HC = 22.1±2.8 |
Whole Brain, Multi-Voxel Analysis Emotional Semantics Task Machine Learning |
1,3Left Inferior Parietal Region and Left IFG predict SI group membership; Gaussian Naïve Bayes classifier correctly discriminated ideators from controls as well as attempters (subset of ideators) from ideators with high (85–94%) accuracy |
| Miller et al., 2018 (85) | SI = 14 No SI = 35 |
13–20 | Whole Brain Emotion Regulation Task Contrast: Reappraisal Negative vs. View Negative; View Negative vs. View Neutral |
1,3SI > No-SI ↑ right DLPFC during cognitive reappraisal; SI > No-SI ↓ Right Thalamus, Left Cerebellum/Lateral Occipital Region, Right DLPFC, Right TPJ (only when controlling for depression symptoms) when viewing negative stimuli |
| Oppenheimer et al., 2020 (80) | ANX = 36 | 11–16 | Whole Brain, ROI (AI and dACC) Chatroom Interact Task Contrast: Rejection vs. Acceptance |
2,4Peer victimization moderated Right AI activation and SI |
| Pan et al., 2011b (78) | MDD+SA = 15 MDD = 15 HC = 14 |
13–17 | Whole Brain Go/No-Go Task |
2,4MDD+SA > MDD ↓ Right Anterior Cingulate Gyrus during response inhibition blocks |
| Pan, Hassel, et al., 2013b (83) | MDD+SA = 14 MDD = 15 HC = 15 |
*MDD+SA = 16.2±0.8 *MDD = 15.9±1.6 *HC = 15.3±1.4 |
Whole Brain, PPI (Right Anterior Cingulate seed) Facial Emotion Processing Task Contrast: Angry 50% Intensity vs. Fixation |
2,4MDD+SA > MDD ↑ Attentional Control Circuitry (Right Anterior Cingulate Gyrus, Left DLPFC), Primary Sensory Cortex, Right MTG; MDD+SA > MDD, HC ↓ PPI to Bilateral Insula connectivity |
| Pan, Segreti, et al., 2013b (77) | MDD+SA = 15 MDD = 14 HC = 13 |
12–17 | Whole Brain Iowa Gambling Task |
2,4MDD > MDD+SA, HC ↑ Left Hippocampus during low-risk decisions (MDD > MDD+SA does not survive post-hoc correction for multiple comparisons) |
| Quevedo et al., 2016a (74) | DEP+HS = 43 DEP+LS = 39 HC = 37 |
11–18 | Whole Brain Emotional Self-Other Morph-Query Task Contrast: Self or Other vs. Baseline for Happy, Neutral, or Sad |
1,4DEP+HS > DEP+LS, HC ↑ Bilateral Cuneus, MOG; DEP+HS, DEP+LS > HC ↑ Right DLPFC across all conditions |
| Non-Suicidal Self-Injury | ||||
| Bonenberger et al., 2015 (99) |
§NSSI = 14 HC = 16 |
*22.4±3.4 | Whole Brain, ROI (Posterior, Medial, and Anterior Insula, Somatosensory Cortex) Electrical Stimulation Task |
1,5NSSI > HC ↓ intensity-modulated activity in AI |
| Brown et al., 2017 (90) | MDD+BPD+NSSIAdult = 14 MDD+NSSIAdolescent = 13 HCAdolescent = 15 HCAdult = 17 |
*MDD+BPD+NSSI = 23.6±4.1 *MDD+NSSI = 15.5±2.0 *HCAdolescent = 14.5±1.7 *HCAdult = 23.2±4.4 |
Whole Brain Cyberball Task Contrast: Exclusion vs. Inclusion |
1,3MDD+BPD+NSSI > HCAdult and MDD+NSSI > HCAdolescent ↑ Ventral ACC; MDD+NSSI > MDD+BPD+NSSI, HCAdolescent ↑ Putamen |
| Demers et al., 2019c (97) | §NSSI = 25 | 13–21 | Whole Brain Masked Emotional Face Task Contrast: Masked Fearful vs. Masked Happy |
1,5↑ Emotional awareness ↑ Right SMA and Right IFG activity |
| Groschwitz et al., 2016 (88) | MDD+NSSI = 14 MDD = 14 HC = 15 |
*15.2±1.8 | Whole Brain, ROI (VLPFC, mPFC) Cyberball Task Contrast: Exclusion vs. Inclusion |
1,3MDD+NSSI > MDD ↑ mPFC, VLPFC, Parahippocampus |
| Osuch et al., 2014 (98) |
§NSSI = 13 §PC = 15 |
16–24 | Task Connectivity, Whole Brain Cold Stimulus Task Contrast: Experimenter or Self, Instruction or Administration of Cool or Cold Stimuli vs. Fixation |
1,3NSSI > PC ↑ Parahippocampal Gyrus, IFG, Amyg, Right Midbrain/Pons, Right Middle Frontal Gyrus across all conditions |
| Perini et al., 2019 (89) |
§NSSI = 27 HC = 27 |
15–18 | Whole Brain, Multi-Voxel Pattern Analysis Social Processing Task |
1,5Whole Brain gray matter, multivariate analysis resulted in 68% accuracy for classification |
| Plener et al., 2012 (95) |
§NSSI = 9 HC = 9 |
14–18 | Whole Brain Emotional and Self-Injuring Image Task |
1,5NSSI > HC ↑ OFC, Inferior Parietal Cortex, Inferior/Middle Frontal Cortex to NSSI stimuli |
| Poon et al., 2019 (93) |
§NSSI-HR = 19 §NSSI-LR = 52 |
12–14 | ROI (Caudate, Putamen, NAcc, vmPFC) Card Guessing Task Contrast: Win vs. Neutral |
2,4NSSI-HR > NSSI-LR ↑ Bilateral Putamen after reward receipt |
| Quevedo et al., 2016 (87) | DEP+NSSI = 50 DEP = 36 HC = 37 |
*14.8±1.6 | Whole Brain Interpersonal Self-Processing Task Contrast: Perspective vs. Baseline |
1,3DEP+NSSI > DEP and HC ↑ Dorsal PFC, Precuneus, PCC, Superior Parietal Lobule, Left/Right Limbic Structures, Fusiform, MTG across all conditions; DEP+NSSI > DEP, HC ↑ Amyg, Hippocampus, Parahippocampus, Fusiform during mother’s perspective |
| Sauder et al., 2016 (92) |
§Self-Injury = 19 HC = 19 |
13–19 | ROI (Striatum, OFC, Amyg) Monetary Incentive Delay Task Contrast: Reward Cue vs. Neutral Cue |
1,5Self-injury > HC ↓ Putamen, OFC, Amyg to reward cues |
| Schreiner et al., 2017c,e (96) |
§NSSI = 24 HC = 17 |
13–21 | Task Connectivity, PPI (Amy seed) Emotional Face Matching Task |
1,5NSSI > HC ↑ Right Amyg connectivity clusters: (a) Right Lingual Gyrus, Occipital Pole, Occipital/Temporal Fusiform, (b) Right Lateral Occipital Cortex, Superior Parietal Lobule during entire task |
Note. Bold = Strong methodological approach (i.e., clinical-control comparison, interview assessments of STB/NSSI, data analysis, large sample)
Age range not reported;.
Overlapping sample
Also includes structural MRI (Table 1)
Also includes resting state MRI (Table 3)
Comorbid disorders see Table S2 for inclusion and exclusion criteria
Age effects not reported
Age effects null
Sex effects not reported
Sex effects null
Female only sample Group.
ANX = Anxiety Disorders; BP = Bipolar Disorder; BPD = Borderline Personality Disorder; DEP = Depressive disorder including MDD, Dysthymia, or Depressive Disorder NOS; HC = Healthy Control; HR = High Risk; HS = High Suicidality, LS = Low Suicidality; MDD = Major Depressive Disorder; NSSI = Non-Suicidal Self-Injury; NSSI-HR = High risk for NSSI based on thoughts; NSSI-LR = Low risk for NSSI based on absence of thoughts; PC = Psychiatric Controls with no NSSI history; SA = Suicide Attempt History; Self-Injury = Self-inflicted injury including SA; SI = Suicide Ideation
Method. PPI = Psychophysiological Interaction; ROI = Region of Interest
Region. ACC = Anterior Cingulate Cortex; AI = Anterior Insula; Amyg = Amygdala; dACC = Dorsal Anterior Cingulate Cortex; DMPFC = Dorsomedial Prefrontal Cortex; DLPFC = Dorsolateral Prefrontal Cortex; IFG = Inferior Frontal Gyrus; IPL = Inferior Parietal Lobule; MFG = Middle Fontal Gyrus; MOG = Middle Occipital Gyrus; mPFC = Medial Prefrontal Cortex; MTG = Middle Temporal Gyrus; NAcc = Nucleus Accumbens; OFC = Orbitofrontal Cortex; PCC = Posterior Cingulate Cortex; PFC = Prefrontal Cortex; SFG = Superior Frontal Gyrus; SMA = Supramarginal Gyrus; TPJ = Temporoparietal Junction; VLPFC = Ventrolateral Prefrontal Cortex; VPFC = Ventral Prefrontal Cortex
Suicidal Thoughts and Behaviors
Self-Processing
Distorted self-referential thinking is a hallmark of adolescent MDD (72), and these biases potentiate suicidal thinking (73). Two recent studies focus on processing images of one’s own versus others’ faces—a subdomain of self-referential processing. Quevedo and colleagues compare adolescents reporting low and high suicidality—composite scores of ideation and attempts. High suicidality youth exhibit greater activation in bilateral cuneus and middle occipital gyrus compared to low suicidality and healthy participants across all conditions. Additionally, both suicidality groups show greater activation in the right DLPFC (74). Within the same sample, high ideators and attempters also show greater task-connectivity between the amygdala and the DLPFC, dACC, dorsomedial PFC, and precuneus compared to low ideators (75). Amygdala-seed PPI connectivity is elevated in the high ideators and attempters relative to the low ideation group in the DLPFC, dorsomedial PFC, and precuneus, and by contrast, connectivity with inferior parietal lobule is elevated in the high ideation group compared to low ideation and attempters. Greater connectivity with the rostral ACC during self versus other face trials also is found for the left amygdala in attempters (compared to all groups) but right amygdala in high ideators (relative to low ideators and controls) (75).
Impulsivity and Social Reward Processes
Impulsive decision-making and experiencing social rejection are known correlates of suicidal behaviors (21, 76). Although non-attempters show greater left hippocampal activation during low-risk (but not high-risk) decisions relative to healthy adolescents and suicide attempters during an Iowa Gambling Task (77), it does not survive post-hoc correction for multiple comparisons. Healthy and attempter youth do not differ. The investigators also administer a response inhibition task, and non-attempters exhibit greater activation within the right ACC relative to attempters but not healthy controls (78).
One study uses the Chatroom Interact Task (79) to probe neural response following social acceptance and rejection. Experience of peer victimization moderates the relationship between right anterior insula activation (rejection versus acceptance) and suicide ideation (80). A study using the Cyberball Task (81) finds that, compared to low ideators and controls, high ideators (including attempters) show reduced activation in the insula and putamen across conditions, but subsequent analyses indicate a pattern of increased activation in the ACC, superior frontal gyrus, and middle frontal gyrus among attempters versus ideators (82).
Emotion Processing
Pan and colleagues test response to face stimuli morphed to different levels of emotional intensity. The most consistent findings emerge during the 50% angry face condition; relative to non-attempters, attempters show greater activation in attentional control circuitry (right ACC, left DLPFC), primary sensory cortex, and right middle temporal gyrus (83). Group differences in PPI show reduced connectivity between a right ACC seed and the bilateral insula when viewing 50% angry faces versus baseline for depressed attempters relative to depressed non-attempters and healthy controls. Recent work from the same research group, however, fails to replicate these effects (84).
During a gender-judgement emotional face task, BD attempters exhibit reduced bilateral amygdala connectivity to the left VPFC (across happy and neutral conditions) and to the right rostral PFC for neutral faces versus baseline. Within the attempter group, suicidal ideation negatively correlates with amygdala-right rostral PFC connectivity, and attempt lethality negatively correlates with amygdala-left VPFC connectivity (53). Among adolescent ideators, there is evidence of greater right DLPFC activation during effortful regulation when viewing negative images compared to non-ideators. There is, however, reduced activation among ideators during passive viewing of negative stimuli within the right thalamus, left cerebellum/lateral occipital region, right DLPFC, and the right temporal parietal junction (85).
One study utilizes machine learning to establish a biological, neurocognitive basis for altered representations of suicide (e.g., death) and emotion (e.g., gloom) words. During the presentation of each word, participants are asked to actively think about the construct. Findings show distinct neural signatures in the left inferior parietal region and left IFG that are predictive of suicide ideator group membership (86). A similar classification approach also discriminates suicide ideators and attempters. A critical next step is to determine whether these neural signatures can identify ideators prior to the attempt.
Non-Suicidal Self-Injury
Self-Processing
Quevedo and colleagues’ task requires participants to use their own (direct) or others’ (indirect: mother, best friend, classmate) perspective when considering self-characteristics (87). Relative to a combined sample of depressed and healthy adolescents, depressed self-injurers exhibit greater activation across all perspectives in the dorsal PFC, precuneus, PCC, superior parietal lobule, left/right temporal limbic structures (amygdala, parahippocampus, hippocampus), fusiform, and middle temporal gyrus. The mother perspective condition (compared to baseline) yields similar findings among self-injurers in the amygdala, hippocampus, parahippocampus, and fusiform.
Social (Reward) Processing
Several NSSI studies probe neural response to social exclusion during the Cyberball Task. Relative to depressed adolescents, depressed self-injurers show increased activation in the medial PFC, ventrolateral PFC, and parahippocampus during exclusion versus inclusion trials; suggesting that self-injurers may view social exclusion more negatively (88) (c.f., (89)). In a subsequent study among the self-injuring youth, there is enhanced activation in the left putamen within the same contrast relative to BPD adults and healthy youth (90).
Research also explores alterations in incentive processing given known associations in adolescent MDD (91). Compared to healthy adolescents, there is blunted reward anticipation in the putamen, OFC, and amygdala in self-injuring youth (92). Among youth at high risk for NSSI (based on thoughts) but without any history of self-injurious behaviors, there is greater bilateral putamen activation following reward receipt compared to youth at low-risk for NSSI, suggesting hypersensitivity to reward (93). Although alterations in anticipatory and consummatory reward processing may underlie risk for NSSI, divergent associations between mental disorders and reward responsiveness (e.g., MDD (91) vs. ADHD (94)) may influence this effect.
Emotion Processing
Researchers use a variety of emotion processing tasks to investigate neural alterations in NSSI. Plener and colleagues show self-injurers and healthy youth IAPS and self-injury images (e.g., razors). Compared to healthy youth, self-injurers exhibit greater activation in OFC, inferior parietal cortex, and inferior/middle frontal cortex to NSSI stimuli (95). During an emotion face-matching task, there is no evidence of emotion-specific effects. However, NSSI and healthy youth show divergent amygdala task-connectivity, but it is not clear whether this reflects NSSI or MDD (or other comorbid disorders) (96). Finally, Demers and colleagues find associations in self-injurers’ emotional awareness and response to viewing masked fearful faces (relative to happy faces) in the supramarginal gyrus and right IFG (97).
Pain Processing
When comparing psychiatric controls to self-injurers, a painful cold compress is either self-administered (analogue to self-injury) or experimenter-administered. Compared to psychiatric controls, self-injurers show greater activation in the parahippocampal gyrus, IFG, and amygdala across conditions (98). Bonenberger and colleagues also probe pain via unpleasant electrical stimulation. Healthy controls exhibit anterior insula activation varying with increasing electrical stimuli intensity, but among self-injurers, this association is blunted (99).
Resting State MRI
The resting state MRI literature on adolescent STB and NSSI is smaller relative to structural and task-based fMRI, and interpretations are challenging given mixed methods and results (Table 3). Investigators primarily apply seed-based approaches, which focus on timeseries correlations between an ROI and the rest of the brain voxel-wise. Several studies apply data-driven approaches (e.g., independent components analysis [ICA]) to explore commonly assessed networks (e.g., default mode network [DMN]). Two studies use voxel-based approaches to characterize local connectivity or timeseries variability.
Suicidal Thoughts and Behaviors
Seed-based
Cullen and colleagues identify depression-related differences in amygdala seed-based resting state functional connectivity (RSFC), but this is not related to suicide severity (100). However, using an expanded sample of depressed youth, within-group analyses show that suicide ideation severity associates with greater connectivity between a right precuneus seed and cerebellum and right IFG, as well as lower connectivity between a left PCC seed and cerebellum and cingulate (101).
Network-based
Compared to psychiatric controls, attempters show increased connectivity between an ICA-derived DMN component and the cerebellum and lingual gyrus, and decreased connectivity with precuneus. Across ICA networks, however, there is reduced positive RSFC between the anterior portion of the DMN and the salience network (SN), less negative anterior DMN-right fronto-parietal network connectivity, as well as increased right FPN-SN and DMN-cerebellum connectivity in patients with versus without an attempt history (102, 103). Among depressed adolescents, lower average connectivity/coherence in the left fronto-parietal executive control network (ECN), anterior DMN, and SN associates with greater lifetime ideation severity (104). Within this same sample, increasing SN coherence predicts lower ideation severity over time (105).
Voxel-wise
Young adults with a lifetime attempt history but no current mental disorders show greater regional homogeneity (ReHo; measuring local synchrony) in the middle frontal gyrus (MFG), parietal lobe, and precuneus, and reduced ReHo in the cerebellum, fusiform, hippocampus, parahippocampal gyrus, angular gyrus, IFG, and MFG compared to healthy controls (106). Cao and colleagues reanalyzed data (102, 103) to examine fractional amplitude of low-frequency fluctuation (ALFF), which characterizes voxel-wise intensity of spontaneous BOLD signal fluctuations across a low-frequency band. Depressed patients with an attempt history show increased ALFF in the medial temporal, superior temporal, and middle occipital gyri compared to patients and healthy controls (107).
Non-Suicidal Self-Injury
Relative to healthy females, adolescents with an NSSI history exhibit reduced left amygdala-seed RSFC with a cluster spanning ACC and supramarginal gyrus (SMA), as well as increased RSFC with angular gyrus and a temporal gyrus cluster (96). These youth underwent an 8-week open-label N-acetylcysteine trial. Reductions in NSSI frequency after treatment associate with decreases in left amygdala-right SMA and right nucleus accumbens-left superior medial frontal cortex RSFC, and increases in right amygdala-right inferior frontal cortex RSFC (108). Finally, relative to healthy adolescents, youth with an NSSI history show reduced amygdala-seed connectivity with the ACC and a cluster spanning right planum temporale and right insula, as well as reduced medial PFC connectivity with the precentral/postcentral gyri and left insula. Reductions in NSSI frequency post-treatment associate with greater negative amygdala-ACC, greater amygdala-brainstem/parahippocampal gyrus, reduced positive medial PFC-amygdala/striatum, and more negative medial PFC-intracalcarine cortex RSFC. Medial PFC-amygdala connectivity also correlates with change in suicidal ideation but not suicidal behavior following treatment (109). Although limited, findings suggest that amygdala-seed connectivity is altered in adolescents with an NSSI history, particularly with the ACC (96, 109).
Discussion
Neural alterations in youth with lifetime STB and NSSI history show modest convergence. First, reduced VPFC and OFC volume associates with STB (53–55), and there is reduced volume in the ACC for youth reporting STB (59) and NSSI (64, 69). Second, blunted striatal activation characterizes STB (82) and NSSI (92), and there is reduced frontolimbic connectivity in suicide ideators and attempters (75). Last, RSFC shows reduced positive connectivity between the DMN and SN in attempters (102, 103), and for NSSI behaviors, growing evidence implicates frontolimbic alterations (96). As a whole, self-injurious behaviors are related to top-down and bottom-up neural alterations.
The overlap in neural correlates of suicidal and non-suicidal behaviors is not surprising given the high co-occurrence (1). However, the inability to elucidate specific neural circuitry may reflect: (i) sample overlap (i.e., NSSI youth in STB studies; STB youth in NSSI studies) and (ii) inconsistent reporting of both STB and NSSI histories (Table S1–S3 for overlap, reporting). Nonetheless, this review highlights tentative differences—focusing on research applying strong methodological approaches (Tables 1–3 bolded publications)—that may prove fruitful to explore. Consistent with Schmaal and colleagues (32), youth STB research is characterized by alterations within prefrontal (53–55) and limbic (62, 75, 102, 103) systems whereby a compromised regulatory system may potentiate suicidal thinking, and perturbations in top-down and bottom-up connections may lead to suicidal behaviors. A key difference is that disruptions across regulatory, approach, and avoidance systems may be even more pronounced in youth given ongoing PFC neuromaturation through young adulthood (30, 31). By contrast, NSSI research reveals ACC structural alterations (64, 69) as well as disruptions in amygdala and ACC connectivity (96), which contributes to hyperarousal and reduced impulse control—central features of NSSI (110, 111). Whether this neural pathway is unique to NSSI remains unclear, as STB research also shows ACC (59) and limbic (75) alterations, and NSSI research has not clarified which ACC (69) and amygdala (96) subregions are more closely linked to NSSI.
Neurodevelopmental Model of STB and NSSI
This review highlights promising neural diatheses related to STB and NSSI. These diatheses are shaped by distal factors occurring during a critical period of neuromaturation (30, 31). Specific genes (e.g., 5-HTTLPR; (112, 113)) influencing neuromaturation have known associations with self-injurious behaviors (114, 115). Trauma and maltreatment occurring during developmentally sensitive periods impact cortical and subcortical development (116), directly affecting top-down (e.g., ACC) and bottom-up (e.g., striatum) processes implicated in STB and NSSI (117). This may be compounded by environment (e.g., poverty (118)), parent (e.g., mental disorders (119)), and youth (e.g., stress (120); psychiatric symptoms (121)) factors—each affecting neuromaturation—and potentially, increasing STB and NSSI vulnerability.
In the context of proximal antecedents, these neural alterations may confer increased STB and NSSI risk (Figure 2). For example, the timing of puberty is important, as early onset for girls versus late onset for boys may increase risk for internalizing disorders (122). Earlier mental disorder onset shapes brain development (e.g., (123)) and relates to self-injurious behaviors (1). Additionally, adolescent interpersonal stress (e.g., loss (21, 25), peer victimization (22, 24))—may initially act on cortical regions by compromising cognitive flexibility and future oriented thinking (124), which over time, may potentiate suicidal and non-suicidal thinking (125, 126). Repeated and chronic stress may tax limbic systems that modulate arousal and approach behaviors, disrupt bottom-up and top-down connections, and potentially, facilitate the transition from ideation to behavior (32, 127).
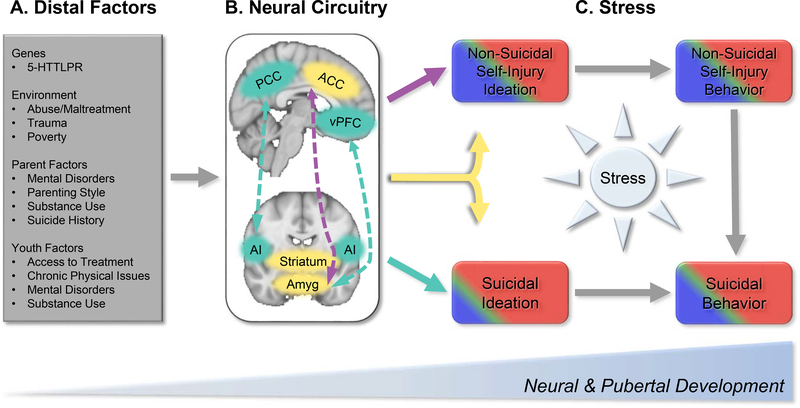
Figure 2.
The neurodevelopmental model of STB and NSSI highlights distal and proximal processes that may potentiate risk for self-injurious behaviors during a critical period of development. There are a wide range of (A) distal risk factors that shape neuromaturation, including genetic, environment, parental, and youth factors. These distal factors occur in the context of ongoing pubertal and neuromaturation from childhood to young adulthood. This review highlights a number of brain regions and connectivity patterns (B) that show alterations potentially unique to suicidal thoughts and behaviors (STB; Teal) and non-suicidal self-injury (NSSI; Purple), or common across both (Yellow). In STB, these include connections between the default mode network (hub in the posterior cingulate cortex [PCC]) and salience network (hub in the anterior insula [AI]) as well as between the ventrolateral prefrontal cortex (VLPFC) and the amygdala (amyg). NSSI shows alterations in connections between the anterior cingulate cortex (ACC) and amygdala. Both STB and NSSI implicate structural and functional alterations in the striatum, amygdala, and ACC. (C) Coupled with acute stressors—particularly interpersonal stress—these distal neural markers may increase risk for engaging in suicidal and non-suicidal behaviors. Acute stress may directly impact brain development, and concurrently, may disrupt top-down cortical processes related to self-referential processing rumination, and future-oriented thinking, which may lead to suicidal and non-suicidal thinking. Stressors that become chronic in nature may tax limbic systems, which modulate arousal and approach behaviors. Disruptions to bottom-up and top-down connections may, for some, facilitate the transition from thinking to acting. More broadly, stress also elicits a range of negative emotions (e.g., sadness, anger), and in the absence of effective emotion regulation strategies, this may then lead to STB and/or NSSI. Presently, there is not sufficient evidence attributing sex differences in STB and NSSI to discrete neural circuitry. Epidemiological research, however, shows that NSSI thoughts and behaviors are more common in female (red) versus male (blue) adolescents, though estimates vary (e.g., (6, 7)). Similarly, suicidal thinking (15% vs. 9%) and behaviors (6% vs. 2%) are more common in females versus males (e.g., (4)). Although not often examined in large epidemiological cohorts, NSSI and STB is proportionally higher among transgender and gender-non-conforming youth (green) (140, 141). Accounting for stress exposure using interview and ambulatory approaches may shed key insights into shared and unique neural markers that lead to suicidal versus non-suicidal behaviors.
Although sex differences in STB and NSSI are well documented (4, 5, 7), there is insufficient evidence showing discrete neural circuitry underlying these effects. One possibility is that neural diatheses do not differ, but rather, stress exposure may differentially trigger underlying diatheses. For example, MDD females report a higher frequency of interpersonal stress (128) whereas males experience more achievement-related stressors (129). The neurobiology of MDD may be similar (91), but the antecedent trigger may differ. Research incorporating stress-based interviews (21) and ambulatory approaches (e.g., experience sampling (130), passive sensor data (131)) may clarify how the type, intensity, and timing of stress exposure impact self-injurious behaviors.
Recommendations for Future Research
Future research addressing key methodological limitations may be better positioned to elucidate unique neural pathways for suicidal and non-suicidal behaviors. Herein, we provide five core recommendations. First, reporting lifetime mental disorders and associated comorbidity will clarify whether effects can be attributed to the targeted behaviors or the underlying disorders. Interview-based assessments of mental disorders, STB, and NSSI are preferable. Self-reports are prone to misattributions and retrospective biases (132), and interviews ensure shared definitions of what constitutes a suicide attempt or an NSSI episode. Given the high co-occurrence of STB and NSSI (1), consistently reporting suicidal and non-suicidal behaviors is essential.
Second, comparing STB or NSSI samples with healthy individuals makes it challenging to attribute neural alterations to self-injurious behaviors versus mental disorders. Comparing suicide attempters versus ideators or adolescents reporting NSSI behaviors versus psychiatric controls may clarify whether there are neural mechanisms implicated in thinking versus engaging in self-injurious behaviors (133).
Third, small samples are ill-equipped to explore age, sex, and gender effects (2, 3; Table S1–S3 for sample sex characteristics). Larger, diverse samples are key given the inherent heterogeneity (e.g., frequency, method, underlying disorders, medication use) and is consistent with initiatives to replicate brain-based effects in representative youth samples (134).
Fourth, longitudinal research is scant. A substantial number of participants characterized by suicidal and non-suicidal thoughts will engage in self-injurious behaviors over time. Longitudinal research—particularly studies incorporating fine-grained ambulatory stress assessments (38)—will have an opportunity to identify distal neural markers that may facilitate the transition from thinking to acting in the context of proximal stressors. This methodological approach can clarify why certain individuals who ideate about suicide or initiate non-suicidal behaviors transition to suicidal behaviors (131).
Fifth, additional neuroimaging modalities—including diffusion tensor imaging (56, 135) and electrophysiology (136, 137)—may provide complementary information. There also may be innovative ways to integrate neuroimaging modalities (e.g., (138, 139) and apply novel data analytic strategies (86).
Summary
Initial research probing neural correlates of STB and NSSI has identified promising brain-based markers. From a neurodevelopmental perspective, an important next step is to elucidate pathways differentially leading to suicidal versus non-suicidal behaviors by incorporating multimodal neuroimaging approaches in the context of fine-grained stress assessments while accounting for wide ranging distal risk factors that influence neuromaturation. Longitudinal research following youth through this developmental period may reveal neural circuitry that potentiates risk for self-injurious behaviors and provide novel targets for treatment.
Supplementary Material
Acknowledgements
Randy P. Auerbach was partially supported by the National Institute of Mental Health (U01MH108168; R01MH119771; R56 MH121426) and the Tommy Fuss Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or NIMH.
Footnotes
For studies assessing parasuicidal behaviors, we use this term to be consistent with findings reported in the published work.
Disclosures
Dr. Auerbach serves on the Research Grants Committee of the American Foundation for Suicide Prevention. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glenn CR, Lanzillo EC, Esposito EC, Santee AC, Nock MK, Auerbach RP (2017): Examining the Course of Suicidal and Nonsuicidal Self-Injurious Thoughts and Behaviors in Outpatient and Inpatient Adolescents. J Abnorm Child Psychol. 45:971–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beauchaine TP, Hinshaw SP, Bridge JA (2019): Nonsuicidal self-injury and suicidal behaviors in girls: the case for targeted prevention in preadolescence. Clinical psychological science. 7:643–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crowell SE, Derbidge CM, Beauchaine TP (2014): Developmental approaches to understanding suicidal and self-injurious behaviors. [Google Scholar]
- 4.Nock MK, Green JG, Hwang I, McLaughlin KA, Sampson NA, Zaslavsky AM, et al. (2013): Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA psychiatry. 70:300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKean AJ, Pabbati CP, Geske JR, Bostwick JM (2018): Rethinking lethality in youth suicide attempts: first suicide attempt outcomes in youth ages 10 to 24. Journal of the American Academy of Child & Adolescent Psychiatry. 57:786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monto MA, McRee N, Deryck FS (2018): Nonsuicidal self-injury among a representative sample of US adolescents, 2015. American journal of public health. 108:1042–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swannell SV, Martin GE, Page A, Hasking P, St John NJ (2014): Prevalence of nonsuicidal self-injury in nonclinical samples: Systematic review, meta-analysis and meta-regression. Suicide and Life-Threatening Behavior. 44:273–303. [DOI] [PubMed] [Google Scholar]
- 8.Klonsky ED, May AM, Glenn CR (2013): The relationship between nonsuicidal self-injury and attempted suicide: Converging evidence from four samples. J Abnorm Psychol. 122:231. [DOI] [PubMed] [Google Scholar]
- 9.Prinstein MJ, Nock MK, Simon V, Aikins JW, Cheah CS, Spirito A (2008): Longitudinal trajectories and predictors of adolescent suicidal ideation and attempts following inpatient hospitalization. J Consult Clin Psychol. 76:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shneidman ES (2001): Comprehending suicide. Landmarks in 20th-century suicidology. [Google Scholar]
- 11.Van Orden KA, Witte TK, Cukrowicz KC, Braithwaite SR, Selby EA, Joiner TE Jr (2010): The interpersonal theory of suicide. Psychol Rev. 117:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nock MK, Prinstein MJ (2004): A functional approach to the assessment of self-mutilative behavior. J Consult Clin Psychol. 72:885. [DOI] [PubMed] [Google Scholar]
- 13.Nock MK (2008): Actions speak louder than words: An elaborated theoretical model of the social functions of self-injury and other harmful behaviors. Applied and Preventive Psychology. 12:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plener PL, Schumacher TS, Munz LM, Groschwitz RC (2015): The longitudinal course of non-suicidal self-injury and deliberate self-harm: a systematic review of the literature. Borderline personality disorder and emotion dysregulation. 2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Séguin M, Beauchamp G, Robert M, DiMambro M, Turecki G (2014): Developmental model of suicide trajectories. The British Journal of Psychiatry. 205:120–126. [DOI] [PubMed] [Google Scholar]
- 16.Borges G, Angst J, Nock MK, Ruscio AM, Kessler RC (2008): Risk factors for the incidence and persistence of suicide-related outcomes: a 10-year follow-up study using the National Comorbidity Surveys. J Affect Disord. 105:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franklin JC, Ribeiro JD, Fox KR, Bentley KH, Kleiman EM, Huang X, et al. (2016): Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. [DOI] [PubMed] [Google Scholar]
- 18.Nock MK (2009): Why do people hurt themselves? New insights into the nature and functions of self-injury. Curr Dir Psychol Sci. 18:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nock MK, Prinstein MJ (2005): Contextual features and behavioral functions of self-mutilation among adolescents. J Abnorm Psychol. 114:140. [DOI] [PubMed] [Google Scholar]
- 20.van Heeringen K (2012): Stress-diathesis model of suicidal behavior. The neurobiological basis of suicide. 51:113. [Google Scholar]
- 21.Stewart JG, Shields GS, Esposito EC, Cosby EA, Allen NB, Slavich GM, et al. (2019): Life stress and suicide in adolescents. J Abnorm Child Psychol. 47:1707–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart JG, Valeri L, Esposito EC, Auerbach RP (2018): Peer victimization and suicidal thoughts and behaviors in depressed adolescents. J Abnorm Child Psychol. 46:581–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slavich GM, Auerbach RP (2018): Stress and its sequelae: Depression, suicide, inflammation, and physical illness APA handbook of psychopathology: Psychopathology: Understanding, assessing, and treating adult mental disorders, Vol 1: American Psychological Association, pp 375–402. [Google Scholar]
- 24.Vergara GA, Stewart JG, Cosby EA, Lincoln SH, Auerbach RP (2019): Non-suicidal self-injury and suicide in depressed adolescents: Impact of peer victimization and bullying. J Affect Disord. 245:744–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prinstein MJ, Guerry JD, Browne CB, Rancourt D (2009): Interpersonal models of nonsuicidal self-injury In Nock MK (Ed.), Understanding nonsuicidal self-injury: Origins, assessment, and treatment (p. 79–98). American Psychological Association. [Google Scholar]
- 26.Tatnell R, Kelada L, Hasking P, Martin G (2014): Longitudinal analysis of adolescent NSSI: The role of intrapersonal and interpersonal factors. J Abnorm Child Psychol. 42:885–896. [DOI] [PubMed] [Google Scholar]
- 27.Spear LP (2000): The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews. 24:417–463. [DOI] [PubMed] [Google Scholar]
- 28.Ernst M, Pine DS, Hardin M (2006): Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 36:299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casey B, Jones RM, Somerville LH (2011): Braking and accelerating of the adolescent brain. Journal of Research on Adolescence. 21:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casey BJ, Jones RM, Hare TA (2008): The adolescent brain. Ann N Y Acad Sci. 1124:111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casey BJ, Getz S, Galvan A (2008): The adolescent brain. Dev Rev. 28:62–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmaal L, van Harmelen AL, Chatzi V, Lippard ETC, Toenders YJ, Averill LA, et al. (2020): Imaging suicidal thoughts and behaviors: a comprehensive review of 2 decades of neuroimaging studies. Mol Psychiatry. 25:408–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guyer AE, Choate VR, Pine DS, Nelson EE (2012): Neural circuitry underlying affective response to peer feedback in adolescence. Soc Cogn Affect Neurosci. 7:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fowler CH, Miernicki ME, Rudolph KD, Telzer EH (2017): Disrupted amygdala-prefrontal connectivity during emotion regulation links stress-reactive rumination and adolescent depressive symptoms. Developmental cognitive neuroscience. 27:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blakemore S-J, Robbins TW (2012): Decision-making in the adolescent brain. Nat Neurosci. 15:1184. [DOI] [PubMed] [Google Scholar]
- 36.Pizzagalli DA (2014): Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 10:393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Heeringen K, Mann JJ (2014): The neurobiology of suicide. The Lancet Psychiatry. 1:63–72. [DOI] [PubMed] [Google Scholar]
- 38.Miller AB, Prinstein MJ (2019): Adolescent suicide as a failure of acute stress-response systems. Annu Rev Clin Psychol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groschwitz RC, Plener PL (2012): The neurobiology of non-suicidal self-injury (NSSI): A review. Suicidology Online. 3:24–32. [Google Scholar]
- 40.Prinstein MJ, Giletta M (2016): Peer relations and developmental psychopathology. Developmental psychopathology. 1–53. [Google Scholar]
- 41.Rudolph KD (2014): Puberty as a developmental context of risk for psychopathology Handbook of developmental psychopathology: Springer, pp 331–354. [Google Scholar]
- 42.Rose AJ, Rudolph KD (2006): A review of sex differences in peer relationship processes: potential trade-offs for the emotional and behavioral development of girls and boys. Psychol Bull. 132:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller AB, Linthicum KP, Helms SW, Giletta M, Rudolph KD, Hastings PD, et al. (2018): Reciprocal associations between adolescent girls’ chronic interpersonal stress and nonsuicidal self-injury: A multi-wave prospective investigation. Journal of Adolescent Health. 63:694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nesi J, Choukas-Bradley S, Prinstein MJ (2018): Transformation of adolescent peer relations in the social media context: Part 1—A theoretical framework and application to dyadic peer relationships. Clin Child Fam Psychol Rev. 21:267–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balcioglu YH, Kose S (2018): Neural substrates of suicide and suicidal behaviour: from a neuroimaging perspective. Psychiatry and Clinical Psychopharmacology. 28:314–328. [Google Scholar]
- 46.Bani-Fatemi A, Tasmim S, Graff-Guerrero A, Gerretsen P, Strauss J, Kolla N, et al. (2018): Structural and functional alterations of the suicidal brain: An updated review of neuroimaging studies. Psychiatry Res Neuroimaging. 278:77–91. [DOI] [PubMed] [Google Scholar]
- 47.Dominguez-Baleon C, Gutierrez-Mondragon LF, Campos-Gonzalez AI, Renteria ME (2018): Neuroimaging Studies of Suicidal Behavior and Non-suicidal Self-Injury in Psychiatric Patients: A Systematic Review. Front Psychiatry. 9:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jollant F, Lawrence NL, Olie E, Guillaume S, Courtet P (2011): The suicidal mind and brain: a review of neuropsychological and neuroimaging studies. World J Biol Psychiatry. 12:319–339. [DOI] [PubMed] [Google Scholar]
- 49.van Heeringen K, Bijttebier S, Desmyter S, Vervaet M, Baeken C (2014): Is there a neuroanatomical basis of the vulnerability to suicidal behavior? A coordinate-based meta-analysis of structural and functional MRI studies. Frontiers in human neuroscience. 8:824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schreiner MW, Klimes-Dougan B, Begnel ED, Cullen KR (2015): Conceptualizing the neurobiology of non-suicidal self-injury from the perspective of the Research Domain Criteria Project. Neuroscience & Biobehavioral Reviews. 57:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ochsner KN, Gross JJ (2005): The cognitive control of emotion. Trends Cogn Sci. 9:242–249. [DOI] [PubMed] [Google Scholar]
- 52.Mitchell DG (2011): The nexus between decision making and emotion regulation: a review of convergent neurocognitive substrates. Behavioural brain research. 217:215–231. [DOI] [PubMed] [Google Scholar]
- 53.Johnston JAY, Wang F, Liu J, Blond BN, Wallace A, Liu J, et al. (2017): Multimodal Neuroimaging of Frontolimbic Structure and Function Associated With Suicide Attempts in Adolescents and Young Adults With Bipolar Disorder. Am J Psychiatry. 174:667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fan S, Lippard ETC, Sankar A, Wallace A, Johnston JAY, Wang F, et al. (2019): Gray and white matter differences in adolescents and young adults with prior suicide attempts across bipolar and major depressive disorders. J Affect Disord. 245:1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huber RS, Subramaniam P, Kondo DG, Shi X, Renshaw PF, Yurgelun-Todd DA (2019): Reduced lateral orbitofrontal cortex volume and suicide behavior in youth with bipolar disorder. Bipolar Disord. 21:321–329. [DOI] [PubMed] [Google Scholar]
- 56.Lippard ETC, Johnston JAY, Spencer L, Quatrano S, Fan S, Sankar A, et al. (2019): Preliminary examination of gray and white matter structure and longitudinal structural changes in frontal systems associated with future suicide attempts in adolescents and young adults with mood disorders. J Affect Disord. 245:1139–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fradkin Y, Khadka S, Bessette KL, Stevens MC (2017): The relationship of impulsivity and cortical thickness in depressed and non-depressed adolescents. Brain Imaging Behav. 11:1515–1525. [DOI] [PubMed] [Google Scholar]
- 58.Ho TC, Cichocki AC, Gifuni AJ, Catalina Camacho M, Ordaz SJ, Singh MK, et al. (2018): Reduced dorsal striatal gray matter volume predicts implicit suicidal ideation in adolescents. Soc Cogn Affect Neurosci. 13:1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goodman M, Hazlett EA, Avedon JB, Siever DR, Chu KW, New AS (2011): Anterior cingulate volume reduction in adolescents with borderline personality disorder and comorbid major depression. J Psychiatr Res. 45:803–807. [DOI] [PubMed] [Google Scholar]
- 60.McLellan Q, Wilkes TC, Swansburg R, Jaworska N, Langevin LM, MacMaster FP (2018): History of suicide attempt and right superior temporal gyrus volume in youth with treatment-resistant major depressive disorder. J Affect Disord. 239:291–294. [DOI] [PubMed] [Google Scholar]
- 61.Pan LA, Ramos L, Segreti A, Brent DA, Phillips ML (2015): Right superior temporal gyrus volume in adolescents with a history of suicide attempt. Br J Psychiatry. 206:339–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beauchaine TP, Sauder CL, Derbidge CM, Uyeji LL (2019): Self-injuring adolescent girls exhibit insular cortex volumetric abnormalities that are similar to those seen in adults with borderline personality disorder. Dev Psychopathol. 31:1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Craig AD (2009): How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 10:59–70. [DOI] [PubMed] [Google Scholar]
- 64.Ando A, Reichl C, Scheu F, Bykova A, Parzer P, Resch F, et al. (2018): Regional grey matter volume reduction in adolescents engaging in non-suicidal self-injury. Psychiatry Res Neuroimaging. 280:48–55. [DOI] [PubMed] [Google Scholar]
- 65.Takahashi T, Chanen AM, Wood SJ, Yucel M, Tanino R, Suzuki M, et al. (2009): Insular cortex volume and impulsivity in teenagers with first-presentation borderline personality disorder. Prog Neuropsychopharmacol Biol Psychiatry. 33:1395–1400. [DOI] [PubMed] [Google Scholar]
- 66.Chanen AM, Velakoulis D, Carison K, Gaunson K, Wood SJ, Yuen HP, et al. (2008): Orbitofrontal, amygdala and hippocampal volumes in teenagers with first-presentation borderline personality disorder. Psychiatry Res. 163:116–125. [DOI] [PubMed] [Google Scholar]
- 67.Takahashi T, Chanen AM, Wood SJ, Walterfang M, Harding IH, Yucel M, et al. (2009): Midline brain structures in teenagers with first-presentation borderline personality disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 33:842–846. [DOI] [PubMed] [Google Scholar]
- 68.Takahashi T, Chanen AM, Wood SJ, Yucel M, Kawasaki Y, McGorry PD, et al. (2010): Superior temporal gyrus volume in teenagers with first-presentation borderline personality disorder. Psychiatry Res. 182:73–76. [DOI] [PubMed] [Google Scholar]
- 69.Whittle S, Chanen AM, Fornito A, McGorry PD, Pantelis C, Yucel M (2009): Anterior cingulate volume in adolescents with first-presentation borderline personality disorder. Psychiatry Res. 172:155–160. [DOI] [PubMed] [Google Scholar]
- 70.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ (1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 6:218–229. [DOI] [PubMed] [Google Scholar]
- 71.McLaren DG, Ries ML, Xu G, Johnson SC (2012): A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 61:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Auerbach RP, Stanton CH, Proudfit GH, Pizzagalli DA (2015): Self-referential processing in depressed adolescents: A high-density event-related potential study. J Abnorm Psychol. 124:233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burke TA, Connolly SL, Hamilton JL, Stange JP, Abramson LY, Alloy LB (2016): Cognitive Risk and Protective Factors for Suicidal Ideation: A Two Year Longitudinal Study in Adolescence. J Abnorm Child Psychol. 44:1145–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quevedo K, Ng R, Scott H, Martin J, Smyda G, Keener M, et al. (2016): The neurobiology of self-face recognition in depressed adolescents with low or high suicidality. J Abnorm Psychol. 125:1185–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alarcon G, Sauder M, Teoh JY, Forbes EE, Quevedo K (2019): Amygdala Functional Connectivity During Self-Face Processing in Depressed Adolescents With Recent Suicide Attempt. J Am Acad Child Adolesc Psychiatry. 58:221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Auerbach RP, Stewart JG, Johnson SL (2017): Impulsivity and suicidality in adolescent inpatients. J Abnorm Child Psychol. 45:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pan L, Segreti A, Almeida J, Jollant F, Lawrence N, Brent D, et al. (2013): Preserved hippocampal function during learning in the context of risk in adolescent suicide attempt. Psychiatry Res. 211:112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pan LA, Batezati-Alves SC, Almeida JR, Segreti A, Akkal D, Hassel S, et al. (2011): Dissociable patterns of neural activity during response inhibition in depressed adolescents with and without suicidal behavior. J Am Acad Child Adolesc Psychiatry. 50:602–611 e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Silk JS, Siegle GJ, Lee KH, Nelson EE, Stroud LR, Dahl RE (2014): Increased neural response to peer rejection associated with adolescent depression and pubertal development. Soc Cogn Affect Neurosci. 9:1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oppenheimer CW, Silk JS, Lee KH, Dahl RE, Forbes E, Ryan N, et al. (2020): Suicidal Ideation Among Anxious Youth: A Preliminary Investigation of the Role of Neural Processing of Social Rejection in Interaction with Real World Negative Social Experiences. Child Psychiatry Hum Dev. 51:163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Williams KD, Cheung CK, Choi W (2000): Cyberostracism: effects of being ignored over the Internet. J Pers Soc Psychol. 79:748–762. [DOI] [PubMed] [Google Scholar]
- 82.Harms MB, Casement MD, Teoh JY, Ruiz S, Scott H, Wedan R, et al. (2019): Adolescent suicide attempts and ideation are linked to brain function during peer interactions. Psychiatry Res Neuroimaging. 289:1–9. [DOI] [PubMed] [Google Scholar]
- 83.Pan LA, Hassel S, Segreti AM, Nau SA, Brent DA, Phillips ML (2013): Differential patterns of activity and functional connectivity in emotion processing neural circuitry to angry and happy faces in adolescents with and without suicide attempt. Psychol Med. 43:2129–2142. [DOI] [PubMed] [Google Scholar]
- 84.Chase HW, Segreti AM, Fournier JC, Phillips ML, Brent D, Pan L Prefrontal BOLD Responses Coupled to Changing Emotional Faces in Adolescents with and without a History of Suicide Attempt. Journal of Medical Psychology.1–10. [Google Scholar]
- 85.Miller AB, McLaughlin KA, Busso DS, Brueck S, Peverill M, Sheridan MA (2018): Neural Correlates of Emotion Regulation and Adolescent Suicidal Ideation. Biol Psychiatry Cogn Neurosci Neuroimaging. 3:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Just MA, Pan L, Cherkassky VL, McMakin DL, Cha C, Nock MK, et al. (2017): Machine learning of neural representations of suicide and emotion concepts identifies suicidal youth. Nat Hum Behav. 1:911–919. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Quevedo K, Martin J, Scott H, Smyda G, Pfeifer JH (2016): The neurobiology of self-knowledge in depressed and self-injurious youth. Psychiatry Res Neuroimaging. 254:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Groschwitz RC, Plener PL, Groen G, Bonenberger M, Abler B (2016): Differential neural processing of social exclusion in adolescents with non-suicidal self-injury: An fMRI study. Psychiatry Res Neuroimaging. 255:43–49. [DOI] [PubMed] [Google Scholar]
- 89.Perini I, Gustafsson PA, Hamilton JP, Kämpe R, Mayo LM, Heilig M, et al. (2019): Brain-based classification of negative social bias in adolescents with nonsuicidal self-injury: findings from simulated online social interaction. EClinicalMedicine. 13:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brown RC, Plener PL, Groen G, Neff D, Bonenberger M, Abler B (2017): Differential Neural Processing of Social Exclusion and Inclusion in Adolescents with Non-Suicidal Self-Injury and Young Adults with Borderline Personality Disorder. Front Psychiatry. 8:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Auerbach RP, Admon R, Pizzagalli DA (2014): Adolescent depression: stress and reward dysfunction. Harv Rev Psychiatry. 22:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sauder CL, Derbidge CM, Beauchaine TP (2016): Neural responses to monetary incentives among self-injuring adolescent girls. Dev Psychopathol. 28:277–291. [DOI] [PubMed] [Google Scholar]
- 93.Poon JA, Thompson JC, Forbes EE, Chaplin TM (2019): Adolescents’ Reward-related Neural Activation: Links to Thoughts of Nonsuicidal Self-Injury. Suicide Life Threat Behav. 49:76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.von Rhein D, Cools R, Zwiers MP, van der Schaaf M, Franke B, Luman M, et al. (2015): Increased neural responses to reward in adolescents and young adults with attention-deficit/hyperactivity disorder and their unaffected siblings. Journal of the American Academy of Child & Adolescent Psychiatry. 54:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Plener PL, Bubalo N, Fladung AK, Ludolph AG, Lule D (2012): Prone to excitement: adolescent females with Non-suicidal self-injury (NSSI) show altered cortical pattern to emotional and NSS-related material. Psychiatry Res. 203:146–152. [DOI] [PubMed] [Google Scholar]
- 96.Schreiner MW, Klimes-Dougan B, Mueller BA, Eberly LE, Reigstad KM, Carstedt PA, et al. (2017): Multi-modal neuroimaging of adolescents with non-suicidal self-injury: Amygdala functional connectivity. J Affect Disord. 221:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Demers LA, Schreiner MW, Hunt RH, Mueller BA, Klimes-Dougan B, Thomas KM, et al. (2019): Alexithymia is associated with neural reactivity to masked emotional faces in adolescents who self-harm. J Affect Disord. 249:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Osuch E, Ford K, Wrath A, Bartha R, Neufeld R (2014): Functional MRI of pain application in youth who engaged in repetitive non-suicidal self-injury vs. psychiatric controls. Psychiatry Res. 223: 104–112. [DOI] [PubMed] [Google Scholar]
- 99.Bonenberger M, Plener PL, Groschwitz RC, Gron G, Abler B (2015): Differential neural processing of unpleasant haptic sensations in somatic and affective partitions of the insula in non-suicidal self-injury (NSSI). Psychiatry Res. 234:298–304. [DOI] [PubMed] [Google Scholar]
- 100.Cullen KR, Westlund MK, Klimes-Dougan B, Mueller BA, Houri A, Eberly LE, et al. (2014): Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry. 71:1138–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schreiner MW, Klimes-Dougan B, Cullen KR (2019): Neural Correlates of Suicidality in Adolescents with Major Depression: Resting-State Functional Connectivity of the Precuneus and Posterior Cingulate Cortex. Suicide Life Threat Behav. 49:899–913. [DOI] [PubMed] [Google Scholar]
- 102.Zhang S, Chen JM, Kuang L, Cao J, Zhang H, Ai M, et al. (2016): Association between abnormal default mode network activity and suicidality in depressed adolescents. BMC Psychiatry. 16:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cao J, Ai M, Chen X, Chen J, Wang W, Kuang L (2020): Altered resting-state functional network connectivity is associated with suicide attempt in young depressed patients. Psychiatry Res. 285: 112713. [DOI] [PubMed] [Google Scholar]
- 104.Ordaz SJ, Goyer MS, Ho TC, Singh MK, Gotlib IH (2018): Network basis of suicidal ideation in depressed adolescents. J Affect Disord. 226:92–99. [DOI] [PubMed] [Google Scholar]
- 105.Schwartz J, Ordaz SJ, Ho TC, Gotlib IH (2019): Longitudinal decreases in suicidal ideation are associated with increases in salience network coherence in depressed adolescents. J Affect Disord. 245:545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cao J, Chen JM, Kuang L, Ai M, Fang WD, Gan Y, et al. (2015): Abnormal regional homogeneity in young adult suicide attempters with no diagnosable psychiatric disorder: a resting state functional magnetic imaging study. Psychiatry Res. 231:95–102. [DOI] [PubMed] [Google Scholar]
- 107.Cao J, Chen X, Chen J, Ai M, Gan Y, Wang W, et al. (2016): Resting-state functional MRI of abnormal baseline brain activity in young depressed patients with and without suicidal behavior. J Affect Disord. 205:252–263. [DOI] [PubMed] [Google Scholar]
- 108.Cullen KR, Schreiner MW, Klimes-Dougan B, Eberly LE, LaRiviere L, Lim KO, et al. (2019): Neural correlates of clinical improvement in response to N-acetylcysteine in adolescents with non-suicidal self-injury. Prog Neuropsychopharmacol Biol Psychiatry. 109778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Santamarina-Perez P, Romero S, Mendez I, Leslie SM, Packer MM, Sugranyes G, et al. (2019): Fronto-Limbic Connectivity as a Predictor of Improvement in Nonsuicidal Self-Injury in Adolescents Following Psychotherapy. J Child Adolesc Psychopharmacol. 29:456–465. [DOI] [PubMed] [Google Scholar]
- 110.Nock MK, Mendes WB (2008): Physiological arousal, distress tolerance, and social problem-solving deficits among adolescent self-injurers. J Consult Clin Psychol. 76:28. [DOI] [PubMed] [Google Scholar]
- 111.Hamza CA, Willoughby T, Heffer T (2015): Impulsivity and nonsuicidal self-injury: A review and meta-analysis. Clin Psychol Rev. 38:13–24. [DOI] [PubMed] [Google Scholar]
- 112.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. (2005): 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 8:828–834. [DOI] [PubMed] [Google Scholar]
- 113.Levitt P, Harvey JA, Friedman E, Simansky K, Murphy EH (1997): New evidence for neurotransmitter influences on brain development. Trends in neurosciences. 20:269–274. [DOI] [PubMed] [Google Scholar]
- 114.Mann JJ, Brent DA, Arango V (2001): The neurobiology and genetics of suicide and attempted suicide: a focus on the serotonergic system. Neuropsychopharmacology. 24:467–477. [DOI] [PubMed] [Google Scholar]
- 115.Cannon DM, Ichise M, Fromm SJ, Nugent AC, Rollis D, Gandhi SK, et al. (2006): Serotonin transporter binding in bipolar disorder assessed using [11C] DASB and positron emission tomography. Biol Psychiatry. 60:207–217. [DOI] [PubMed] [Google Scholar]
- 116.Andersen SL, Teicher MH (2008): Stress, sensitive periods and maturational events in adolescent depression. Trends in neurosciences. 31:183–191. [DOI] [PubMed] [Google Scholar]
- 117.Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, et al. (2006): Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry. 59:975–982. [DOI] [PubMed] [Google Scholar]
- 118.Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, et al. (2013): The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA pediatrics. 167:1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Spadoni AD, Simmons AN, Yang TT, Tapert SF (2013): Family history of alcohol use disorders and neuromaturation: a functional connectivity study with adolescents. The American journal of drug and alcohol abuse. 39:356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Carrion VG, Weems CF, Reiss AL (2007): Stress predicts brain changes in children: a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics. 119:509–516. [DOI] [PubMed] [Google Scholar]
- 121.Muetzel RL, Blanken LM, van der Ende J, El Marroun H, Shaw P, Sudre G, et al. (2018): Tracking brain development and dimensional psychiatric symptoms in children: A longitudinal population-based neuroimaging study. American Journal of psychiatry. 175:54–62. [DOI] [PubMed] [Google Scholar]
- 122.Negriff S, Susman EJ (2011): Pubertal timing, depression, and externalizing problems: A framework, review, and examination of gender differences. Journal of Research on Adolescence. 21:717–746. [Google Scholar]
- 123.Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, et al. (1996): Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 53:607–616. [DOI] [PubMed] [Google Scholar]
- 124.Arnsten AF (2009): Stress signalling pathways that impair prefrontal cortex structure and function. Nature reviews neuroscience. 10:410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Keilp JG, Beers SR, Burke AK, Melhem NM, Oquendo MA, Brent DA, et al. (2014): Neuropsychological deficits in past suicide attempters with varying levels of depression severity. Psychol Med. 44:2965–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stewart JG, Polanco-Roman L, Duarte CS, Auerbach RP (2019): Neurocognitive Processes Implicated in Adolescent Suicidal Thoughts and Behaviors: Applying an RDoC Framework for Conceptualizing Risk. Current Behavioral Neuroscience Reports. 6:188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Oliveira JF, Dias NS, Correia M, Gama-Pereira F, Sardinha VM, Lima A, et al. (2013): Chronic stress disrupts neural coherence between cortico-limbic structures. Frontiers in Neural Circuits. 7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rudolph KD (2002): Gender differences in emotional responses to interpersonal stress during adolescence. Journal of adolescent health. [DOI] [PubMed] [Google Scholar]
- 129.Rudolph KD, Hammen C (1999): Age and gender as determinants of stress exposure, generation, and reactions in youngsters: A transactional perspective. Child Dev. 70:660–677. [DOI] [PubMed] [Google Scholar]
- 130.Glenn CR, Kleiman EM, Kearns JC, Santee AC, Esposito EC, Conwell Y, et al. (2020): Feasibility and acceptability of ecological momentary assessment with high-risk suicidal adolescents following acute psychiatric care. Journal of Clinical Child & Adolescent Psychology. 1–17. [DOI] [PubMed] [Google Scholar]
- 131.Allen NB, Nelson BW, Brent D, Auerbach RP (2019): Short-term prediction of suicidal thoughts and behaviors in adolescents: Can recent developments in technology and computational science provide a breakthrough? J Affect Disord. 250:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Millner AJ, Lee MD, Nock MK (2015): Single-item measurement of suicidal behaviors: Validity and consequences of misclassification. PloS one. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Klonsky ED, May AM (2014): Differentiating suicide attempters from suicide ideators: A critical frontier for suicidology research. Suicide and LifeThreatening Behavior. 44:1–5. [DOI] [PubMed] [Google Scholar]
- 134.Pagliaccio D, Alqueza KL, Marsh R, Auerbach RP (2019): Brain Volume Abnormalities in Youth at High Risk for Depression: Adolescent Brain and Cognitive Development Study. Journal of the American Academy of Child & Adolescent Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schreiner MW, Mueller BA, Klimes-Dougan B, Begnel ED, Fiecus M, Hill D, et al. (2020): White matter microstructure in adolescents and young adults with non-suicidal self-injury. Frontiers in psychiatry. 10:1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tsypes A, Owens M, Gibb BE (2019): Blunted neural reward responsiveness in children with recent suicidal ideation. Clinical Psychological Science. 7:958–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pegg S, Dickey L, Green H, Kujawa A (2020): Differentiating clinically depressed adolescents with and without active suicidality: An examination of neurophysiological and selfreport measures of reward responsiveness. Depress Anxiety. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Siless V, Hubbard NA, Jones R, Wang J, Lo N, Bauer CC, et al. (2020): Image Acquisition and Quality Assurance in the Boston Adolescent Neuroimaging of Depression and Anxiety Study. NeuroImage: Clinical. 102242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hubbard N, Siless V, Frosch I, Goncalves M, Lo N, Wang J, et al. (2020): NBrain Function and Clinical Characterization in the Boston Adolescent Neuroimaging of Depression and Anxiety Study. NeuroImage: Clinical.102240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Veale JF, Watson RJ, Peter T, Saewyc EM (2017): Mental health disparities among Canadian transgender youth. Journal of Adolescent Health. 60:44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu RT, Mustanski B (2012): Suicidal ideation and self-harm in lesbian, gay, bisexual, and transgender youth. American journal of preventive medicine. 42:221–228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.