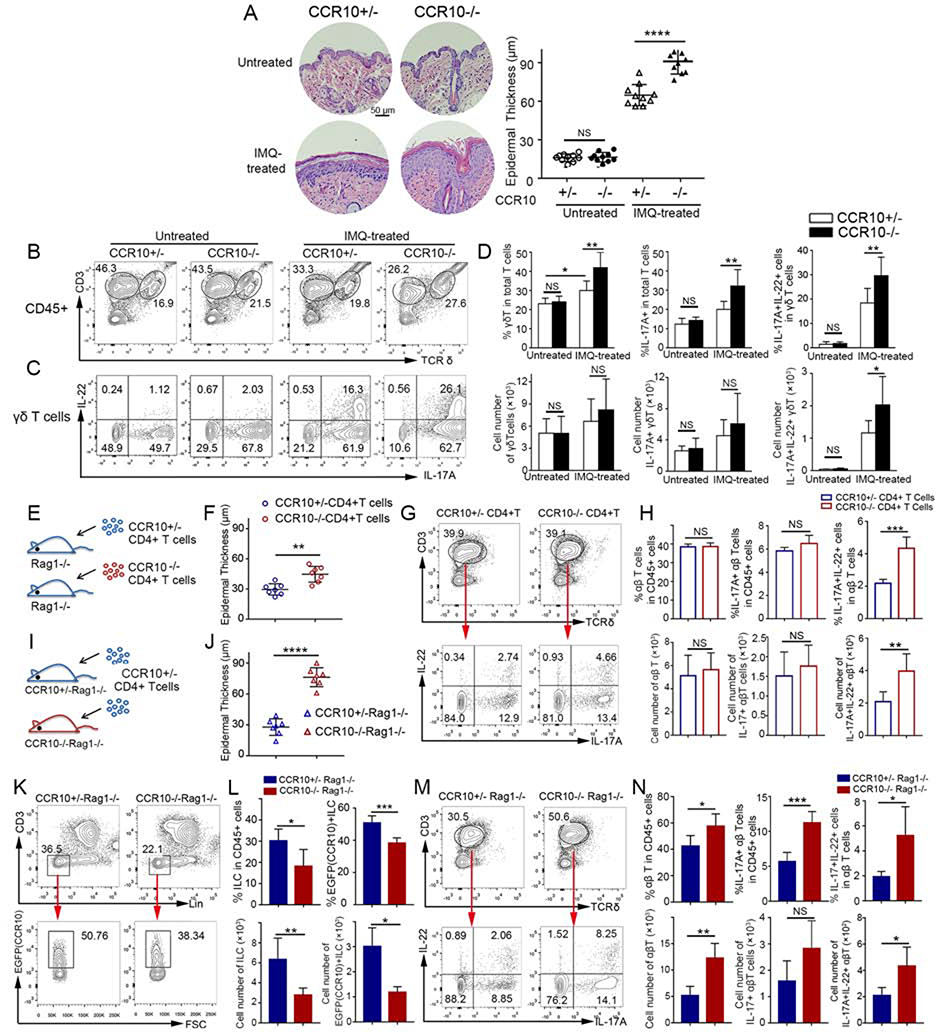
Figure 1.
Enhanced IL-17A/IL-22-producing skin T cell activation and inflammation in IMQ-treated CCR10-knockout mice. (A,B,C,D) H&E stained sections and average epidermal thickness (A), flow cytometric (FC) analysis of γδT cells (B) and their IL-17A/IL-22 production (C), and average percentages and numbers of IL-17A/IL-22+ γδT cells (D) of untreated and 5 day-IMQ-treated skin of CCR10−/− and CCR10+/− mice. (E) Scheme of reconstitution of Rag1−/− mice with CCR10−/− or CCR10+/− CD4+ αβT cells. (F,G,H) Average epidermal thickness (F), FC analysis of donor αβT cells and their IL-17A/IL-22 production (G), and average percentages and numbers of IL-17A/IL-22+ αβT cells (H) of the IMQ-treated skin of Rag1−/− mice reconstituted with CCR10−/− or CCR10+/− CD4+ αβT cells. (I) Scheme of reconstitution of CCR10+/−Rag1−/− or CCR10−/−Rag1−/− mice with same CCR10+/− CD4+ αβT cells. (J,K,L,M,N) Average epidermal thickness (J), FC analysis (K) and percentages and numbers (L) of EGFP(CCR10)+ host ILCs, and FC analysis (M) and percentages and numbers (N) of IL-17A/IL-22+ donor αβT cells of the IMQ-treated skin of CCR10+/−Rag1−/− and CCR10−/−Rag1−/− mice reconstituted with CCR10+/− CD4+ αβT cells. One dot represents one sample (A,F,J). N=5–9 (D) and 5 each (H,L,N). NS: not significant, *P<0.05, **P<0.01, ***P<0.001, ***P<0.0001.