Figure 1.
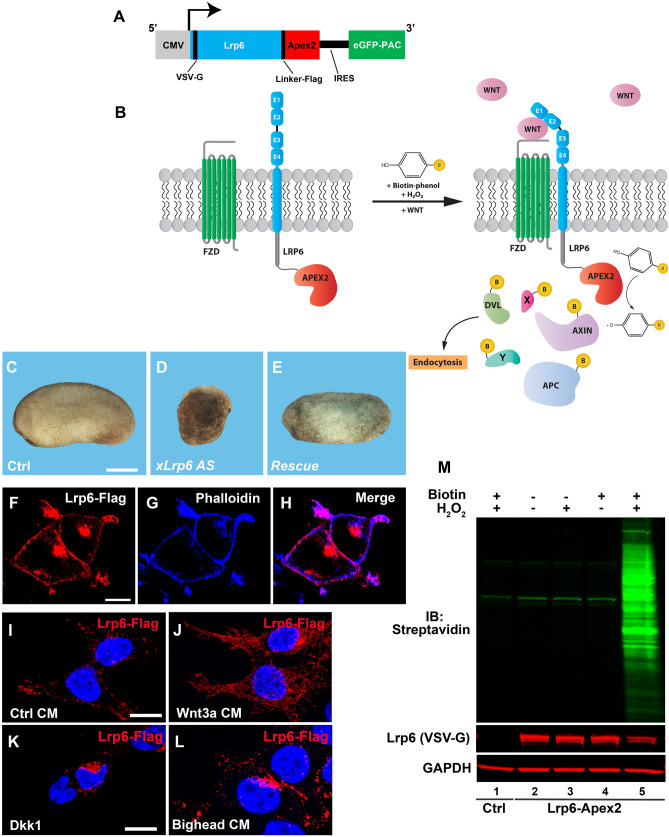
The Lrp6-APEX2 fusion protein receptor retains signal transducing activity, proper subcellular localization, and biotinylates cellular proteins. (A) Schematic diagram of the Lrp6-APEX2 construct. The human Lrp6 contains an N-terminal VSV-G and a linker Flag-tag followed by the APEX2 enzyme. The construct also contains an internal ribosomal entry site (IRES) followed by a GFP-tagged Puromycin N-acetyltransferase (PAC) used for selection of permanently transfected cells. (B) Diagram depicting the biotinylation of Lrp6-APEX2 proximal targets. During the Wnt signal, in the presence of Biotin-phenol (BP) and H2O2, proteins recruited to the receptor are biotinylated by the APEX2 peroxidase, including known Wnt targets (Dvl, Axin, APC, etc.) and novel targets (X, Y). (C–E) control embryos from oocyte-host transfer experiments show normal development (100%, n = 24), as compared to oocytes depleted with a phosphorothioate antisense DNA oligo targeting Xenopus Lrp627, which develop as ventralized embryos (75%, n = 22). Co-injection of 300 pg of Lrp6-APEX2 mRNA into oocytes completely rescued axis formation (60%, n = 17), indicating that the fusion protein is fully active. Scale bar represents 500 µm. (F–H) Lrp6-APEX2 is trafficked to the plasma membrane in stably transfected HEK293T cells. Lrp6 was detected through its Flag-tag and Phalloidin, which stains cortical F-Actin, confirmed cell surface localization of Lrp6. Scale bar represents 10 µm. (I–L) Lrp6-APEX2 changes subcellular localization by Wnt, Dkk1 and Bighead treatments. In presence of control conditioned medium (CM), Lrp6 is located mostly at the plasma membrane (79%). Treatment with Wnt3a CM for 30 min increases the number of intracellular vesicles containing Lrp6 (53%). Treatment with Dkk1 protein (200 ng/ml) or Bighead CM induced relocation of Lrp6 to the juxtanuclear bay area where lysosomes are located (63% and 53%, respectively). Quantification was obtained counting cells showing the observed immunostaining over total nuclei, in 10 different micrographs. Scale bars represent 10 µm. (M) western blot stained with Streptavidin-IRDye 800 (in green) showing controls indicating that Lrp6-APEX2 biotinylates proximal proteins only in the presence of both BP and H2O2 (compare lane 5 with 2–4). Negative control cells not expressing the APEX2 peroxidase had no biotinylation even in presence of BP/H2O2 (lane 1). Note the presence of three Streptavidin-positive bands in all lanes, which correspond to endogenous biotinylated carboxylases. Gapdh served as a loading control.