FIGURE 1.
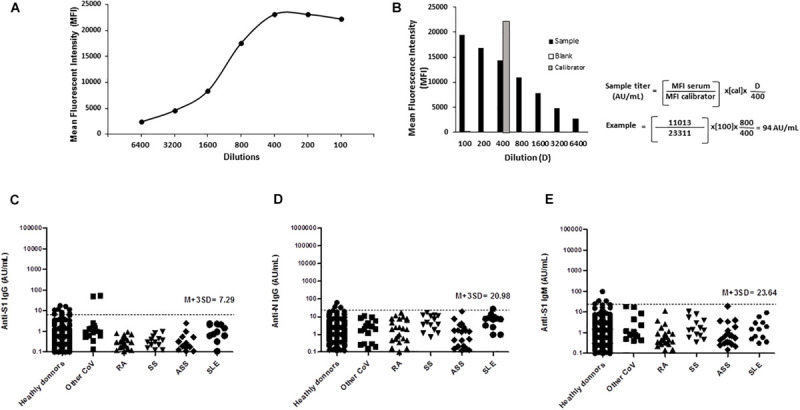
Detection, titration, and cross-reactivity of anti–SARS-CoV-2 Spike S1, nucleocapsid N protein IgG, and anti–SARS-CoV-2 Spike S1 IgM antibodies by ALBIA-IgG-S1/N and ALBIA-IgM-S1. (A) A calibration curve was obtained after serial dilutions of the calibrator, i.e., one highly positive sample. A plateau of MFI was reached for dilutions 1:400 or lower. (B) Calculation of antibody titer by reference to the MFI value of the calibrator (gray bar) used at a 1:400 dilution in the assay and its level arbitrarily set to 100 arbitrary units (AU)/mL. The assay was first performed using a 1:100 screening dilution of the serum. In case the sample’s MFI at 1/100 dilution was higher than 70% of the calibrator’s MFI, further dilutions were performed, and the first dilution yielding an MFI inferior to 70% of calibrator MFI was retained for calculation. An example is given: at 1:100 dilution, the MFI was higher than 70% of the calibrator’s MFI (23,311 × 0.7 = 16,318), requiring a 1/800 dilution for computing the titer, i.e., 94 AU/mL anti-S1 IgG level. Specificity toward non–COVID-19 patients: (C) anti-Spike S1 and (D) anti-N IgG, IgM, and (E) anti-Spike S1 IgM antibody reactivity in patients with different conditions: PCR-confirmed infection with other CoV (17 sera from 13 patients; HKU1, n = 3; OC43, n = 11; NL63, n = 3). RA, rheumatoid arthritis; SS, Sjögren syndrome; ASS, antisynthetase syndrome; SLE, systemic lupus erythematosus.
