Abstract
Loss of central vision can be compensated for in part by increased use of peripheral vision. For example, patients with macular degeneration or those experiencing simulated central vision loss tend to develop eccentric viewing strategies for reading or other visual tasks. The factors driving this learning are still unclear and likely involve complex changes in oculomotor strategies that may differ among people and tasks. Although to date a number of studies have examined reliance on peripheral vision after simulated central vision loss, individual differences in developing peripheral viewing strategies and the extent to which they transfer to untrained tasks have received little attention. Here, we apply a recently published method of characterizing oculomotor strategies after central vision loss to understand the time course of changes in oculomotor strategies through training in 19 healthy individuals with a gaze-contingent display obstructing the central 10° of the visual field. After 10 days of training, we found mean improvements in saccadic re-referencing (the percentage of trials in which the first saccade placed the target outside the scotoma), latency of target acquisition (time interval between target presentation and a saccade putting the target outside the scotoma), and fixation stability. These results are consistent with participants developing compensatory oculomotor strategies as a result of training. However, we also observed substantial individual differences in the formation of eye movement strategies and the extent to which they transferred to an untrained task, likely reflecting both variations in learning rates and patterns of learning. This more complete characterization of peripheral looking strategies and how they change with training may help us understand individual differences in rehabilitation after central vision loss.
Keywords: perceptual learning, central vision loss, oculomotor system, macular degeneration
Introduction
Perceptual learning in the context of central vision loss is a topic of increasing attention. From a societal perspective, there is a substantial need to develop better interventions for patients with central vision loss, such as in macular degeneration (MD), which is projected to affect 248 million people worldwide by 2040 (Wong, Su, Li, Cheung, Klein, Cheng, & Wong, 2014). Although patients with central vision loss often develop compensatory oculomotor strategies to adapt to the loss of central vision, these vary across individuals, and patients often struggle to conduct tasks of daily living such as reading, navigating, finding objects, and recognizing faces. The purpose of this paper is to apply a recently published method of characterizing oculomotor strategies after central vision loss (Maniglia, Visscher, & Seitz, 2020) to understand the time course of perceptual learning of compensatory oculomotor strategies after simulated vision loss and the extent to which these strategies do or do not transfer between the trained task and an untrained task. Our hope is that, by understanding how these oculomotor strategies change through perceptual learning in cases of simulated scotomas, this could pave the way toward the development of interventions that could help train patients to better perform tasks of daily living.
The most common compensatory oculomotor strategy after central vision loss is the development of a preferred retinal locus (PRL), a peripheral location that takes over duties including those requiring high-resolution vision, such as reading and recognizing faces (Cummings, Whittaker, Watson, & Budd, 1985; Fletcher & Schuchard, 1997; Timberlake, Mainster, Peli, Augliere, Essock, & Arend, 1986; Von Noorden & Mackensen, 1962). Often, eye movement planning is altered so that saccades direct the PRL (rather than the fovea) to land on targets, a strategy referred to as saccadic re-referencing. Its effects can be so profound that patients report looking straight ahead when fixating a target with their PRL (White & Bedell, 1990; Whittaker & Cummings, 1990). However, there are substantial inter-individual differences in the location, stability, number, and time course of development of PRLs (Crossland, Culham, Kabanarou, & Rubin, 2005; Fletcher & Schuchard, 1997; Guez, Le Gargasson, Rigaudiere, & O'Regan, 1993; Sunness, Applegate, Haselwood, & Rubin, 1996; White & Bedell, 1990).
From a basic science perspective, the development of PRLs and other compensatory oculomotor strategies represents a dramatic ecological example of perceptual learning. Importantly, recent studies have had success at examining these compensatory strategies by using gaze-contingent displays to simulate central vision loss in normally seeing populations (Barraza-Bernal, Rifai, & Wahl, 2017a; Kwon, Nandy, & Tjan, 2013; Walsh & Liu, 2014). For example, Kwon and colleagues showed that simulated scotomas improved fixation stability and induced re-referencing of eye movements away from the fovea to a trained peripheral locus (Kwon et al., 2013). This use of gaze-contingent displays has unlocked the ability to study perceptual learning in the context of central vision loss in highly controlled laboratory settings.
Although perceptual learning with simulated central vision loss offers a number of advantages with regard to helping us understand the development of peripheral looking strategies that may be informative to ecological cases of central vision loss, numerous methodological and ecological issues must still be addressed before this will serve as a successful model of central vision loss. First, there are several differences between pathological and simulated scotomas. As examples, simulated scotomas are typically uniform across time and have visible boundaries, which may be used as an oculomotor reference to redirect saccades (Van der Stigchel, Bethlehem, Klein, Berendschot, Nijboer, & Dumoulin, 2013; Walsh & Liu, 2014), and those with simulated scotomas experience central vision loss for a short period a day and for just a few handfuls of days. On the other hand, in MD, the size and shape of scotomas change progressively across time, patients are typically unaware of their boundaries, and studied patients often have years of experience with full-time central vision loss. Also, notably PRL development is often slow for MD patients (Crossland et al., 2005) and seems to be much faster in the case of simulated scotoma (Kwon et al., 2013), perhaps due to the visible boundaries of the occluder (Walsh & Liu, 2014). Still, qualitative similarities between studies of peripheral looking strategies in patients with MD and research participants with simulated scotomas abound, including recent evidence that healthy participants trained with asymmetrical scotoma sizes maintain the PRL of the eye with the smaller scotoma (Lei & Chung, 2020), which is consistent with clinical reports in MD patients.
Most studies on simulated (and pathological) central vision loss focus on the overall fixation distribution and its change after training, mostly in terms of PRL location respect to the fovea, area of dispersion (Barraza-Bernal et al., 2017a; Kwon et al., 2013; Walsh & Liu, 2014) and/or performance improvements in the trained location (Liu & Kwon, 2016). To address the possibility of patients exhibiting multiple PRLs, Crossland and colleagues proposed a cluster-based analysis that allows extraction of oculomotor information separately for different PRLs (Crossland, Sims, Galbraith, & Rubin, 2004). Further, to measure re-referencing in healthy participants trained with artificial scotomas, Kwon and colleagues looked at the location of the first saccade landing location in each trial (Kwon et al., 2013). Still, relatively little is known of the finer details of the peripheral viewing strategies as they are developed, the extent to which they transfer to untrained tasks, and whether they develop consistently across individuals. Crucially, most of the current analyses of oculomotor behavior in conditions of (simulated) central vision loss do not differentiate fixations or eye movements from different trials, thus potentially conflating different behaviors within and across trials into a single eye position distribution.
In this paper, we apply a recently published method to characterize oculomotor strategies after central vision loss (Maniglia et al., 2020) to understand the time course of how oculomotor behavior changes through contrast detection training in conditions of simulated central vision loss. By using this new method, we hope to achieve an in-depth classification of eye movements that disentangles a number of peripheral looking behaviors (see Figure 1). This approach allows us to examine eye movement behaviors on individual trials and divide trials on the basis of which PRL was first used (e.g., in which of the PRL locations the first saccade outside the scotoma in each trial landed; see Figure 1, central panel). Additionally, to test the generalization of oculomotor learning to an untrained task, we analyzed changes in visual acuity and eye movement behavior on an untrained task before and after training. Generalization of learning is the main goal of studies that aim at clinical applications (Lu, Lin, & Dosher, 2016); however, how oculomotor learning transfers from one task to another has yet to be studied in conditions of simulated central vision loss.
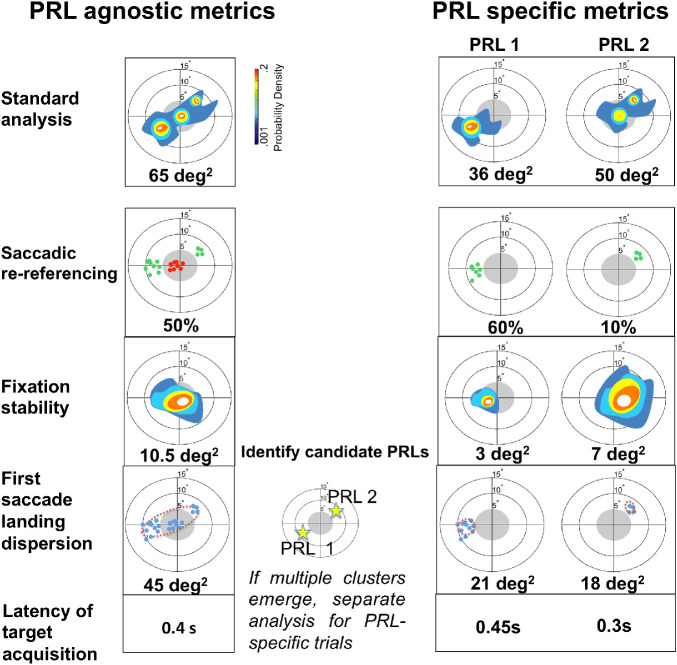
Figure 1.
Summary of the oculomotor metrics used in the study applied to an example pattern of fixation. Oculomotor metrics were first calculated on the whole dataset (PRL agnostic, left column) and, in cases of multiple PRLs, then separately for trials that used each PRL (PRL-specific, right column). In all of the figures, the central gray region extending 5° of radius into the visual field represents the simulated scotoma. In order to identify multiple PRLs, the distribution of the first saccade landing dispersion was evaluated to determine whether fixations outside the scotoma were distributed into multiple clusters, as exemplified in the central panel. If this was the case, then metrics were calculated separately for each cluster. Metrics illustrated are as follows (from top to bottom): Probability density map of the fixation distribution, which is the standard approach used in the literature. In this analysis, a kernel density estimator (KDE) was fitted to the distribution of eye positions, resulting in a color-coded graph revealing clusters of fixations. The metric was then extracted by computing a BCEA encompassing 68% of the total eye position (Crossland et al., 2004). We note that we consider this metric to be illustrative, as it combines all fixations; it is shown here and in the Supplementary Material but is not considered to be a metric of interest for identifying details of learning. Saccadic re-referencing reflects how often participants immediately see the target. It is the proportion of trials where the end point of the first saccade put the target outside the scotoma. The green dots represent first fixations in each trial that successfully placed the target outside the scotoma. Fixation stability reflects whether the eye tends to remain stable on a given trial. It is a within-trial measure of dispersion, and, similar to the standard approach, it utilizes a KDE fitted on the distribution of eye position to visually represent the within-trial oculomotor behavior. The score of this metric was then obtained by computing a BCEA on the distribution. First saccade landing dispersion reflects how precisely saccades are planned. It is an across-trial measure of dispersion of the end point of the first saccade in each trial. Latency of target acquisition reflects how long it takes to observe the target. It is the mean time until the target is visible outside the scotoma.
To address these aims, we trained 19 healthy participants over 10 daily sessions while the central 10° of visual field were obstructed by a gaze-contingent, eye-tracker–generated circular occluder. Participants were trained using one of two protocols: (a) standard perceptual learning (SPL), in which an oriented Gabor patch was always presented in the center of the screen until the participant's response, and (b) coordinated attentional training (CAT), in which the Gabor patch could appear anywhere on screen accompanied by a visual cue to signal its location. In both tasks, the judgment was on the orientation of the Gabor, and difficulty was adaptive by varying the contrast and spatial frequency of the Gabor. However, given the substantial individual differences in oculomotor behavior within each condition, we present the data for each training type but do not delve into differences as a function of training task.
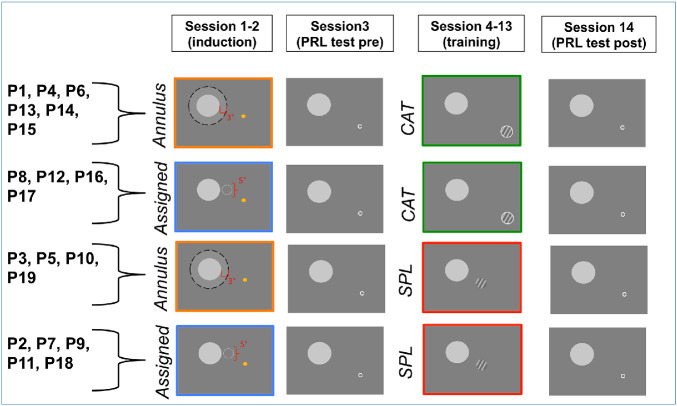
We note that, although Maniglia et al. (2020) used a portion of the same dataset, the goal of that paper was to describe, motivate, and validate the methods for describing oculomotor strategies, and it only considered the post-test data. In the current manuscript, we examine details of eye movement–related metrics as they changed during the course of perceptual learning training and how they differed between training and test sessions. Figure 1 highlights the metrics from Maniglia et al. (2020) that are applied in the current manuscript: first saccade landing dispersion, saccadic re-referencing, fixation stability, and latency of target acquisition. Figure 2 shows the training regime and the different training and testing sessions that are discussed in the current manuscript. Results showed mean improvements in saccadic re-referencing (percentage of trials in which the first saccade placed the target outside the scotoma), latency of target acquisition (time interval between target presentation and a saccade that would put the target outside the scotoma), and fixation stability (within-trial dispersion of eye movements). However, we also observed substantial individual differences in oculomotor strategies within the trained task and in the transfer of these strategies to the untrained task. This finding appears to mirror what is found in the clinical literature, such that effective use of the visual periphery develops inconsistently across individuals, and patients often use differing peripheral strategies across tasks and time-points (Crossland et al., 2005; Fletcher & Schuchard, 1997; Guez et al., 1993; Sunness et al., 1996; White & Bedell, 1990).
Figure 2.
Procedure timeline and assignments to condition. Each participant underwent one of the two induction procedures (assigned PRL or annulus PRL; participant identifiers in each group are listed on the left) and one of the two training conditions (CAT or SPL). Test sessions were conducted identically across all conditions. During induction, participants were asked to identify the emoji (was it smiling or not?). The yellow circle of the emoji was always visible, but its face only appeared when the participant's gaze was oriented so that the emoji fell within a fixed location relative to central vision (assigned condition) or an annulus around the simulated scotoma (annulus condition). The gray disk was a gaze-contingent occluder of central vision, present on the screen. The labels shown in this image were not presented to the participants but serve here to indicate the locations where the face of the emoji would be visible. The contrast detection training tasks (CAT or SPL) are described in the text in the Training section. The PRL test was identical for all participants regardless of training task or induction procedure.
Methods
Participants
Nineteen healthy participants (mean age, 20.4 ± 1.8 years; 12 females, 7 males) with normal or corrected-to-normal vision and no known visual pathologies or cognitive or neurological impairments were recruited at the University of California at Riverside to take part in the study. Experimental protocols were approved by the Human Research Review Board of the University of California at Riverside, and all participants gave written informed consent prior to the experiment. We note that participants were randomly assigned between two different training conditions (CAT or SPL) that were crossed with two different PRL induction conditions (e.g., a pre-training manipulation used to acquaint participants with peripheral viewing and intended to develop an initial PRL). In the assigned PRL condition, the PRL location was chosen for participants by the experimenter, whereas in the annulus condition participants were able to freely develop their own PRL location (see below for more details). This resulted in four different conditions: assigned PRL/SPL (n = 5), assigned PRL/CAT (n = 4), annulus PRL/SPL (n = 4), and annulus PRL/CAT (n = 6) (for more details, see Figure 2). In this manuscript, we do not distinguish between these groups, largely due to a lack of power and the large individual variability, but data in both the manuscript and the Supplementary Material allow readers to come to their own conclusions regarding the impacts of each condition.
Stimuli and apparatus
Participants’ eye movements were monitored (monocular tracking of the right eye) using an infrared video-based eye tracker sampled at 500 Hz (EyeLink 1000 Plus Tower Mount, SR Research Ltd., Ontario, Canada) using drift correction. In order to ensure sufficient precision for each participant, each session started with a nine-point calibration/validation sequence that was repeated until the validation error was smaller than 1° on average. The gaze position error (i.e., difference between the target position and the computed gaze position) was estimated during the nine-point validation procedure. The tower mount chin and forehead rests from the EyeLink system were used throughout the experiment to minimize head movements and trial-to-trial variability in the estimation of gaze position. Real-time gaze positions were sent to the display computer through a high-speed Ethernet link. The monitor was a ViewSonic PF817 Professional Series CRT with a resolution of 1280 × 1024 pixels and a refresh rate of 75 Hz (ViewSonic Corp., Brea, CA). Each pixel subtended 0.032° of visual angle at the viewing distance of 57 cm. The continuous gaze information was used to draw the artificial scotoma on the experimental monitor at a refresh rate of 75 Hz where the gaze position corresponded to the center of the scotoma. The EyeLink 1000 has a worst-case latency of 4 ms, and with the 75-Hz frame rate used and a CRT monitor with almost complete phosphor decay by 2 ms, there was ample time to recompute the stimuli between frames. To verify the system latency, we used the method described in Saunders and Woods (2014). High-frame video recording using a similar setup showed a screen update of about 28 ms (median value of 50 measurements, corresponding to three frames in the worst-case scenario); however, we were unable to test the exact system used for the study due to an ongoing campus shutdown caused by the COVID-19 pandemic. Notably, subjective reports from both the research staff and participants using the experimental setup were that the artificial scotoma appeared to smoothly follow eye movements and that the target could not be made visible to foveal viewing even when making rapid eye movements. Viewing was binocular; however, eye tracking was monocular. Thus, there is a possibility that the untracked eye deviated in some cases from the location of the tracked eye. PRLs developed in many participants nonetheless; however, it may be the case the PRLs would have developed differently if all viewing was monocular.
A digital-to-analog converter (Bits++; Cambridge Research Systems, Rochester, UK) was used to increase the dynamic contrast range (10-bit luminance resolution). A 10-bit gamma-corrected lookup table was used to linearize the luminance of the monitor. The luminance of the artificial scotoma was 11% higher than the luminance of the background display, amounting to 50% (127 RGB) and 39% (100 RGB) of the maximum screen luminance, respectively. The Psychophysics Toolbox (Brainard, 1997; Pelli, 1997) and EyeLinkToolbox (Cornelissen, Peters, & Palmer, 2002) were used to generate visual stimuli.
Procedure
In each session, a gaze-contingent display simulating a 10° (diameter) circular scotoma, obstructing central vision, was used to induce peripheral looking strategies. An overview of the procedure is shown in Figure 2. In sessions 1 and 2, a PRL induction procedure (see below for more details) was used to acquaint participants with peripheral viewing. In sessions 4 to 13, participants experienced the perceptual learning training procedures (see below for details) designed to further develop and strengthen these peripheral looking strategies. In sessions 3 and 14, each participant performed a visual acuity task aimed at measuring the characteristics of their PRL and oculomotor strategies in a transfer task (PRL test session). All sessions lasted ∼45 minutes.
PRL induction
During this phase of familiarization with the artificial scotoma, participants underwent either the assigned PRL or the annulus induction type. In the assigned PRL condition, a circular region of 5° diameter, centered at an eccentricity of 7.5° from the center of the scotoma, was randomly selected for each participant to the left or right of the scotoma, and participants were instructed to put the target within this region for it to be visible. In the annulus PRL condition, the target was visible in a region extending 3° radially from the border of the scotoma. The target consisted of an emoji that, outside the regions of visibility, looked like a yellow circle. The purpose of varying induction type was to determine the extent to which different induction methods give rise to systematically different effects and the extent to which assigned PRLs are maintained when restricting viewing conditions are relaxed. However, large variability within conditions precluded significant conclusions with the current sample size. Consequently, the oculomotor analyses presented here were conducted over the whole sample, but data for each induction type are visually separated in the graphs and additional details are provided in the Supplementary Material.
PRL test
In sessions 3 and 14, participants performed a visual acuity task aimed at measuring visual performance and oculomotor strategies in a transfer task (PRL test session). At the beginning of each trial, participants were presented with a central rectangle slightly larger than the artificial scotoma and asked to center their gaze so the scotoma would be within the boundaries of the rectangle. Then, a Landolt C, in the Sloan font (Pelli, Robson, & Wilkins, 1988) and at 100% luminance (255 RGB), appeared at a random location, anywhere on the screen, and participants were asked to report its orientation (C opens up, down, left, or right). The size of the letter C was initially 2° and progressively increased (decreased) following correct (incorrect) responses according to a 3:1 staircase. The 10° diameter simulated scotoma was present in the display exactly as in the induction sessions. Because of the lesser acuity of peripheral vision, Landolt C stimuli are most visible when close to the fovea (close to the border of the scotoma), thus motivating the participants to make eye movements placing the C near the border of the scotoma. Each PRL test session had 70 trials (∼8 minutes). Visual acuity thresholds were calculated as arithmetic means of the last 20 trials.
Training
During sessions 4 to 13, participants were trained with a contrast detection task where they reported the orientation (left vs. right) of a Gabor patch (σ = 1° of visual angle) that remained visible on the screen until response. Participants were assigned to one of two training conditions (see Figure 2): SPL or CAT. We note that these data are part of a pilot study intended to estimate the effect sizes related to differences in outcomes from SPL versus CAT; however, for the purposes of the present manuscript, data are combined across conditions, as the observed variability of peripheral looking strategies was larger within than across conditions. A larger sample size would be required to make robust differentiations between training conditions, but, for those interested in the per-condition effects, the data are displayed as a function of condition in the figures and comparisons between conditions are provided in the Supplementary Material.
In SPL, the Gabor patch was always presented in the center of the screen accompanied by a neutral auditory cue, meaning that the training did not require searching for the target but only required identifying whether a low contrast target was oriented left or right. On the other hand, during CAT, the target could appear anywhere on screen, requiring a search and reorienting of gaze toward the target. The target was accompanied by a visual cue (a circle around the target) that was either very bright or dim, meaning that on some trials the location was visually salient but on others it was not. Additionally, the target in CAT was accompanied by an auditory cue indicating its position on the screen (through pitch and interaural time/level differences). The auditory cue was panned left or right according to the horizontal position of the target (based on interaural time/level differences), and its pitch was higher or lower depending on the target position along the vertical axis. Thus, although SPL training involved a more standard, static perceptual learning paradigm, CAT incorporated shifts of attention toward different cued locations in space.
In both types of training, contrast and spatial frequency of the Gabor patch were subject to a staircase procedure; specifically, the contrast started from 20% and progressively decreased following correct responses according to a 3:1 staircase (after three correct responses the contrast was decreased by 0.1 log unit, and after one incorrect response it was increased by 0.1 log unit). When a 1% contrast was reached, the spatial frequency, which started at 3 cycles per degree, doubled and the contrast was reset to 20%. Participants received auditory feedback on their performance. During training, the target was always visible and remained on the screen until the participant's response. Participants were instructed to report the orientation of the target as quickly and accurately as possible. Each training session lasted 500 trials (∼45 minutes).
Data analyses
To evaluate changes in oculomotor behavior after training with simulated central vision loss, we used four of the metrics described in Maniglia et al. (2020). We first calculated agnostic metrics (see the Supplementary Material), and then, in cases of multiple clusters of fixations outside the scotoma, we conducted PRL-specific analyses considering the larger cluster (e.g., the main PRL), as shown in Figure 2, right column. Specifically, following Crossland et al. (2004), we determined the number of clusters by visually inspecting the contour plots of the kernel density estimates, with yellow/orange regions indicating separate clusters. We briefly describe the metrics here and refer readers to Maniglia et al. (2020) for more details.
Saccadic re-referencing measures the ability of participants to place the target in a visible position outside the scotoma with the first saccade of each trial and is computed as the percentage of trials in which the first fixation landed outside the scotoma.
Fixation stability is a within-trial measure of the dispersion of eye positions. Similar to previous studies in patients (Crossland et al., 2004) and healthy participants trained with artificial scotoma (Kwon et al., 2013; Liu & Kwon, 2016), we calculated a bivariate contour ellipse area (BCEA) on the eye positions of each participant. In contrast to previous studies, however, we computed a dispersion for each trial and then we normalized this measure by trial duration and averaged it across trials. These across-trial fixation stability distributions are plotted centered at a common reference point: the mean across trials of the center of the BCEA. Thus, for a single PRL, the plots of fixation stability are centered at that PRL location.
First saccade landing dispersion is an across-trial measure of dispersion and is computed as the BCEA of the distribution of the end points of the first saccade in each trial (similar to the re-referencing measure in Kwon et al., 2013).
Latency of target acquisition measures the time interval between target appearance and the first fixation that would put the target in a visible location.
We selected these metrics because they address four crucial questions that involve the use of a PRL: (a) Is there a shift in oculomotor referencing away from the fovea (saccadic re-referencing)? (b) Is this shift localized in a specific peripheral retinal region (first saccade landing dispersion)? (c) How long does it take for the oculomotor system to locate the target (latency of target acquisition)? (d) When a target has been acquired, is there stable fixation (fixation stability)? Although we largely focus on the PRL-specific metrics (Figure 1, right column), in that we considered only trials in which a first fixation landed outside the scotoma, results for agnostic measures are provided in the Supplementary Material.
Results
The organization of the results section is as follows: (a) assess changes in performance (visual acuity) following the training, (b) characterize the changes in oculomotor behavior following the training, (c) assess transfer of learning from the trained condition to a different task, and (d) show the variety of eye movement behavior that can be observed after training. Each of these analyses provides a complementary picture of the perceptual learning that resulted from training normally seeing participants under conditions of simulated scotoma.
Changes in performance (visual acuity)
As a first measure of perceptual learning, we examined whether peripheral visual acuity, an untrained visual ability, improved through training (Figure 3). Overall, acuity improved significantly (paired t-test, t18 = 2.55, p = 0.02). However, a one-way analysis of variance on visual acuity threshold differences between pre- and post-training assessments did not show a significant difference among SPL and CAT and the assigned PRL and annulus induction conditions, F(3, 15) = 0.95, p = 0.441. This lack of differentiation among conditions may be due to either a lack of difference between conditions or merely a lack of power. For the remainder of the manuscript, although plots retain information about the four conditions wherever possible, the main analyses lump across conditions (a breakdown of results by conditions can be found in the Supplementary Material).
Figure 3.
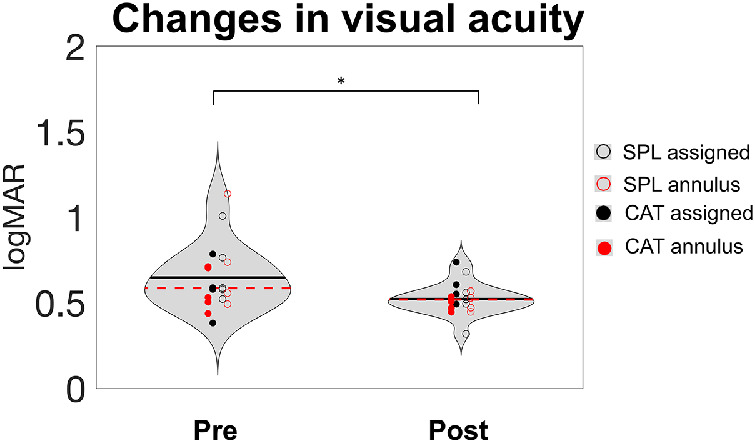
Visual acuity in the Landolt C task. Visual acuity improved significantly with training (p = 0.02, t-test conducted on the whole subject dataset). Data from pre/post PRL test sessions are presented as violin plots. Dashed red lines in each violin represent the median, and the black lines indicate the mean across all participants. Each colored dot represents one of the participants in the four combinations of training and induction procedures.
Changes in oculomotor behavior
We next examined whether metrics of oculomotor behavior changed with training. Figure 4 shows data for each metric of interest, with thin lines showing individual subject data for pre and post tests (colored dots), lines showing linear fits of training data, and panels in each section showing detailed plots of three representative participants. To quantify training data, we fit the data with a linear model and conducted one-sample t-tests against zero on the slope of the fit. Results showed a significant learning effect for saccadic re-referencing (t18 = 3.339, p = 0.004) and latency of target acquisition (t18 = 4.276, p < 0.0001). On the other hand, first saccade landing dispersion showed only a trend (t18 = 2.04, p = 0.056), and fixation stability failed to show a significant change with training (t18 = 0.285, p = 0.779). However, we can also see that there were substantial individual differences, and these are addressed toward the end of the Results section.
Figure 4.
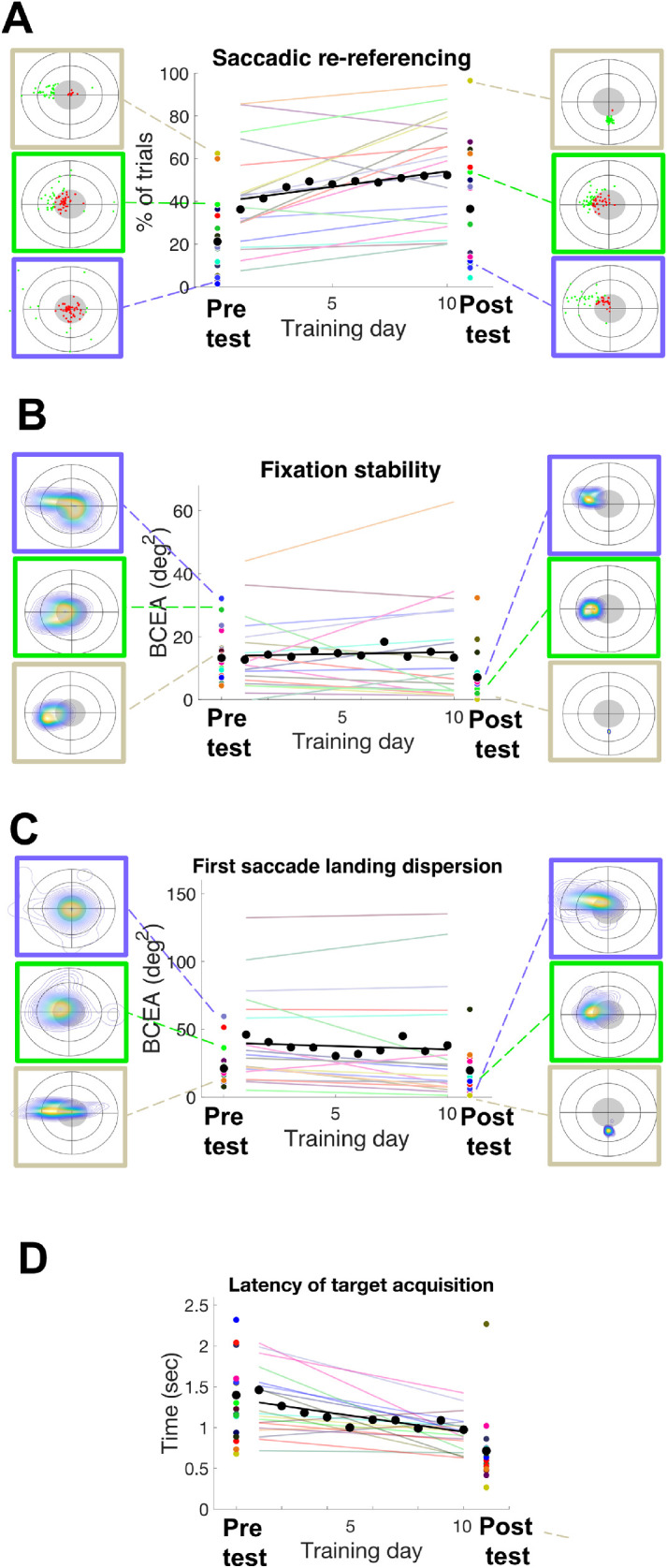
Summary of training effect on oculomotor metrics. Saccadic re-referencing (A), fixation stability (B), first saccade landing dispersion (C), and latency of target acquisition (D) are shown for each testing and training day for each participant. Performance trends for each participant are shown as a linear fit of their training score, in different colored lines for each participant. Pre/post training performance (PRL test) is presented as colored dots. The gray lines and dots represent the average performance for each metric. Boxes in A to C indicate examples of metrics during pre/post tests for three representative participants.
Transfer of learning to untrained task (PRL test)
We next examined whether learned patterns of oculomotor metrics transferred to the untrained visual acuity task. Figure 5A shows changes in oculomotor metrics for both training and transfer task (PRL test), computed as [(post – pre)/pre]*100. For visual clarity and to ensure that the largest number of participants were included in the graphs, in this figure outliers were removed based on a conservative criterion (score >3 SD above the mean). In successive analyses, however, we used a procedure described in Jones (2019), which led to the exclusion of four participants from the transfer analysis. We observed a significant improvement in fixation stability (one-sample t-test against zero, t14 = 9.15, p < 0.0001), as well as significant changes in saccadic re-referencing (t14 = 3.22, p = 0.006) and latency of target acquisition (t14 = 12.16, p < 0.0001). On the other hand, first saccade landing dispersion showed a non-significant effect (t14 = 0.84, p = 0.415). We note, however, that without the removal of outliers significant transfer of learning was found only for saccadic re-referencing (t18 = 3.58, p = 0.002) and latency of target acquisition (t18 = 7.02, p < 0.0001), but not for first saccade landing dispersion (t18 = 0.824, p = 0.421) or fixation stability (t18 = 0.183, p = 0.857). Similar effects were observed when all trials were included (as the PRL agnostic analysis), but are not included here for clarity. The PRL agnostic analyses are reported in the Supplementary Material.
Figure 5.
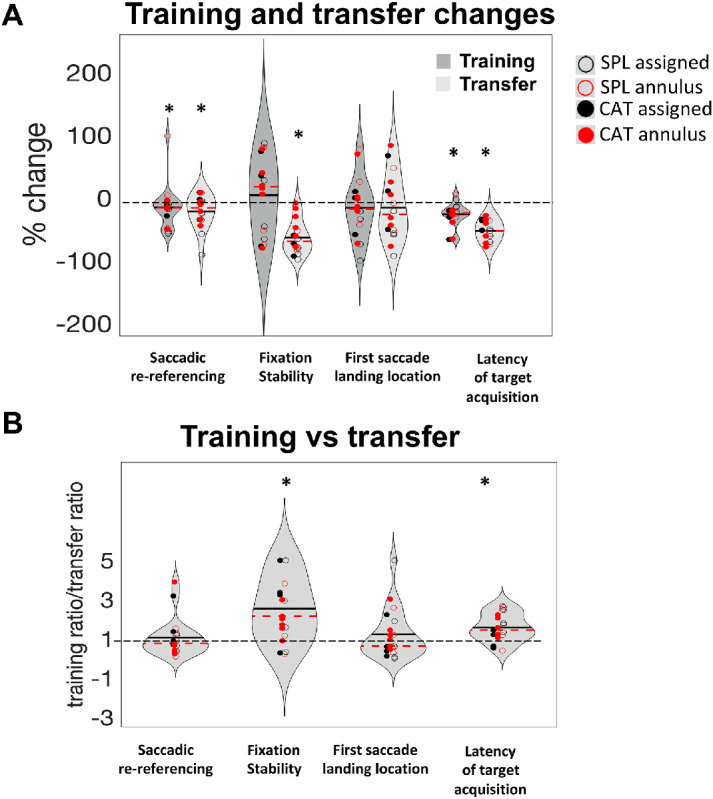
Training and transfer summary results. (A) Percentage change for the four metrics in the training (dark gray) and transfer (light gray) tasks. Differently colored dots represent the four different training-plus-induction conditions described in the Methods section. Dashed red lines in each violin represent the median, and the black lines indicate the mean. Asterisks indicate significant improvements (one-sample t-test vs. zero, α = 0.05). In this analysis (PRL-specific), the PRL with the larger number of useful fixations was chosen for each participant (see Methods), and metrics for that PRL are shown. So that all metrics plotted improvements with training as values below 0 on the graph, saccadic re-referencing (good when higher) was entered into the calculations as 100 – saccadic re-referencing, so that lower values are better. For clarity of graphical representation, three participants (P3, P17, P18) were removed as outliers (scores >3 SD from the mean). (B) Training versus transfer improvement for the four metrics. Ratios larger than one indicate that participants improved more within the pre/post assessment than in the training task; ratios smaller than one indicate that participants improved more in the training task than in the pre/post-assessment. Stars indicate significantly greater improvement in the pre/post assessment than the training task. To avoid small denominators dominating the effect, we capped the ratios at five for the three participants that exhibited ratios greater than five.
Somewhat surprisingly, Figure 5 suggests that there may be a larger transfer effect than training effect. To further quantify this, we computed the ratio of ratios between the training and transfer effects—specifically, (post test score/pre test score)/(session_10 score/session_1 score). In this measure, values greater than 1 indicate that participants improved more in the transfer task than in the training task, a value = 1 indicates equal improvement in training and transfer tasks, and lower values indicate impartial transfer. Surprisingly, larger improvements in the transfer task compared to the training task were observed for latency of target acquisition (t18 = 3.49, p = 0.003) and fixation stability (t18 = 2.44, p = 0.025). On the other hand, the transfer of learning for saccadic re-referencing (t18 = 0.24, p = 0.81) and first saccade landing dispersion (t18 = 0.934, p = 0.363) was equal to the training gain (ratios not significantly different from 1). Although it may seem counterintuitive for there to be more transfer of learning than learning on the training task, a simple explanation may be that there was some improvement between the pre-test and the first training session (see Figure 4), and learning may be incomplete with some additional gains after training session 10. We also note that there were significant individual differences in the transfer effect, which are described in the qualitative analyses below.
Summary training and transfer analysis (principal component analysis)
We conducted a principal component analysis (PCA) to examine change scores for the trained task and the transfer task (PRL test) in order to better describe how participants improved on the training or transfer tasks. Each participant contributed eight values to the analysis: one change score (post test vs. baseline) for each of the four metrics. These were calculated for the trained task (denoted PP, for pre/post), as well as for the transfer task (denoted T). Figure 6 shows a plot of the two principal components, with red dots representing scores for individual participants and blue lines representing coefficients for improvements in each of the eight metrics. The variances explained by the first two principal components were 33.11% and 23.8%, respectively.
Figure 6.
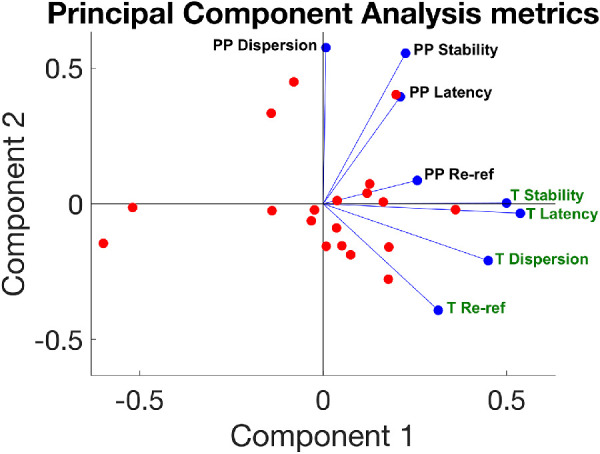
Principal component analysis. The graph shows the distributions along the two main components; individual participants’ scores on these components are plotted as red dots. The weighting of each of the metrics is shown in blue. Change scores for each of the four metrics are calculated for the training task data (labeled “T” and green), as well as for the data during the pre/post tests of transfer (labeled “PP” and black). These metrics are training saccadic re-referencing, training first saccade landing dispersion, training latency of target acquisition, training fixation stability, pre/post saccadic re-referencing, pre/post first saccade landing dispersion, pre/post latency of target acquisition, and pre/post fixation stability.
All of the metrics had the same directionality for component 1, indicating that this component may reflect an overall improvement on all metrics. The metrics calculated for the trained task (green labels) are all lower on the component 2 axis, and the metrics calculated for the PRL test (black labels) are all higher on that axis, thus component 2 differentiated improvements on the training and the transfer tasks. Further, changes in fixation stability and latency of target acquisition seem highly correlated in both training and transfer tasks.
Qualitative analysis
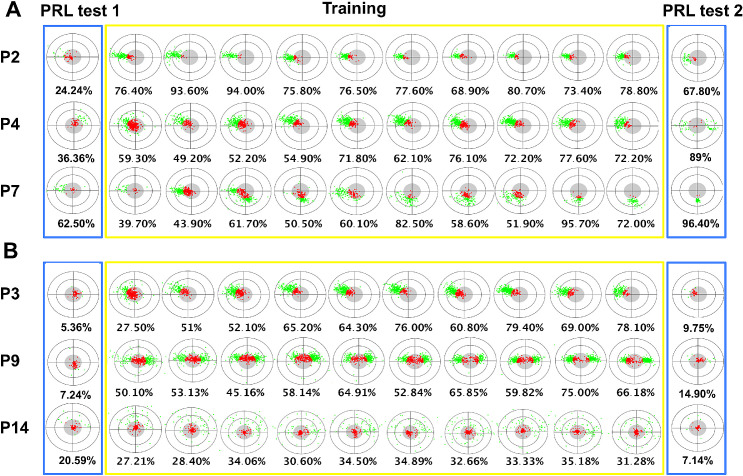
Clinical studies on MD patients are often characterized by high inhomogeneity in the participant sample and large variability in their performance. In this next section, we describe patterns of results in individual participants, which these metrics gave us the opportunity to observe. We keep in mind that these results are purely descriptive; still, however, qualitative examination of the data suggests individual differences that are not fully apparent in the quantitative analyses. In Figure 7, we describe re-referencing for some participants exemplifying the two main patterns of behavior observed. Individual participant scores for each metric and for each day of training are provided in the Supplementary Material to allow the reader to appreciate this variety of behaviors.
Figure 7.
Time course of saccadic re-referencing. (A) Participants P2, P4, and P7 showed different peripheral looking strategies but all had substantial re-referencing and distribution of fixations that were consistent between training and PRL test 2. (B) Participants P3, P9, and P14 again showed different peripheral looking strategies, but these did not transfer to PRL test 2, and all three showed lower referencing in the untrained condition. Data from all participants are provided in Supplementary Figure S1.
An examination of Figure 7 quickly reveals substantial diversity across subjects, such that some developed highly refined PRLs (such as P2) but others show highly scattered patterns of fixations (such as P4). Overall, most participants exhibited peripheral re-referencing, with some notable exceptions such as P14. However, the patterns of fixations in these profiles differed substantially. Many participants developed a single PRL (e.g., P2, P3, P7). Others showed a pattern where first fixations occurred anywhere along the horizontal axis (e.g., P4, P9). Notably, this behavior is consistent with clinical evidence from MD patients, who tend to develop PRLs to the left or to the right of the retinal scotoma (Crossland et al., 2005). Finally, a number of participants, such as P14, did not seem to have a clear strategy. Although the number of PRLs seemed to be established early on during training and was maintained throughout the session, the location of the PRLs seemed to be subject to change with training days for some participants. For example, P7 gradually rotated their PRL across days of training (from left of the scotoma to below).
With regard to the relationship between training and testing sessions, we can qualitatively distinguish two types of training and transfer behaviors—specifically, those who developed a PRL and showed transfer of learning to the untrained task (PRL/transfer group, Figure 7A) and those who did not develop a clear PRL and/or showed reduced or absent transfer of learning (PRL weak/no transfer, Figure 7B). These behaviors appear similarly distributed in our sample of participants (n = 10 vs. 9; see all in the Supplementary Material). It is important to mention that the lack of peripheral re-referencing in some participants might be due to insufficient training; indeed, previous studies showed that eccentric viewing strategies, and in particular re-referencing, can require several more hours of training than the duration of our study (Kwon et al., 2013), meaning that our study is likely focused on eye movement metrics in the midst of training. Although it may be the case that similar strategies would emerge with more experience with the untrained task, these results suggest that for many participants there is a task dependence to their peripheral looking strategies.
Discussion
Altogether, the results presented here confirm previous findings of substantial plasticity through experience with simulated central scotomas, even after just a few hours of experience. Most participants demonstrated substantial saccadic re-referencing (tendency to place the target outside the simulated scotoma with the first saccade of the trial), which increased with training. Training also resulted in reduction in latency of target acquisition (time in between target presentation and a saccade that places it outside the scotoma) and, for the transfer task, improved fixation stability (within-trial ability to maintain stable fixation). We also observed substantial individual differences in both quantitative and qualitative inspections of the data. Although some participants developed well-focused PRLs, others developed horizontal streaks of fixations and others more random patterns. Further, the stability of these within training and also between training and test sessions differed substantially across participants. Although it seems that different phenotypes of peripheral looking strategies are visibly apparent across the metrics, an even larger dataset may be required to clearly quantify these, as well as to determine how they depend on the different training conditions. Overall, the present results may be most helpful in pointing out the diversity of peripheral looking strategies that develop through perceptual training with simulated scotomas and the metrics that can be used to help understand them.
Importantly, we tested for the first time, to the best of our knowledge, whether there was transfer of learning of oculomotor strategies from the trained task (contrast detection) to a different one (visual acuity). On a group level, results show significant improvements in saccadic re-referencing, latency of target acquisition, and fixation stability, consistent with the results in the trained task. The evidence of successful transfer of learning of oculomotor behaviors developed in conditions of simulated central vision loss is consistent with a recent study by Barraza-Bernal, Rifai, and Wahl (2017b) showing PRL location retention between tasks in healthy participants trained with artificial scotoma. Such data represent a promising result in the context of translational science, as they suggest that training in a clinical context could translate to tasks of daily life. However, we do note that this transfer was only evident in a subset of participants, and further research will be required to better understand the reasons why some participants exhibited more or less transfer.
This manuscript is an extension of a recently published methods paper addressing how to characterize oculomotor strategies after central vision loss (Maniglia et al., 2020). Whereas that paper used data from the post test to illuminate how the various metrics are appropriate to characterize different dimensions of peripheral looking strategies, here we have applied some of these metrics to understand the time course of how oculomotor behavior changes through contrast detection training in conditions of simulated central vision loss. We suggest that these metrics can help the field to understand which specific behaviors are related to better behavioral outcomes by correlating changes in specific oculomotor metrics with, for example, changes in visual acuity or contrast sensitivity. Additionally, these approaches might help describe profiles of compensation along multiple dimensions, rather than just re-referencing or fixation stability, as is currently typical. This more detailed approach will be helpful for understanding compensation in low vision populations.
Macular degeneration (MD), for example, is one of the most common visual diseases and an increasingly worrying health concern in Western countries (Wong et al., 2014). These patients tend to spontaneously use a different retinal spot (PRL) outside the damaged foveal region to perform tasks such as fixation and fine detail vision (Fletcher & Schuchard, 1997; Timberlake et al., 1986; Von Noorden & Mackensen, 1962). This use-dependent change, known as re-referencing, is in some cases so profound that it leads patients to report looking straight ahead when they use their PRL for fixation (White & Bedell, 1990; Whittaker & Cummings, 1990). Studies in the field of optometry and vision science have attempted to shed light on these intrinsic adaptive changes and potentially improve residual vision after central vision loss with training (Fine & Rubin, 1999; Maniglia, Pavan, Sato, Contemori, Montemurro, Battaglini, & Casco, 2016; Nilsson, 1990; Plank, Rosengarth, Schmalhofer, Goldhacker, Brandl-Rühle, & Greenlee, 2014; Rosengarth, Keck, Brandl-Rühle, Frolo, Hufendiek, Greenlee, & Plank, 2013). However, issues of a practical nature make research in low-vision populations challenging.
Thus, approaches utilizing simulated central vision loss present a number of practical advantages over their clinical counterpart. Simulated scotoma may provide a platform for testing rehabilitation protocols and simulating visual pathologies at different stages, given the flexibility offered by the simulation of the parameters. Healthy individuals can be trained to spontaneously exhibit some of the compensatory behaviors observed in MD patients (Barraza-Bernal et al., 2017a; Kwon et al., 2013; Walsh & Liu, 2014). However, the field is still in its initial stages, and few aspects of oculomotor behaviors have been examined. Additionally, current methods derived from clinical practice with MD patients can conflate distinct trial-by-trial patterns of behaviors. Although the current study presents a number of limitations, mostly due to the small sample size for each combination of induction and training procedure and the relatively short training period, the use of detailed metrics helps us visualize and understand details of individual peripheral looking strategies and learning trajectories that otherwise would remain opaque.
Of note, one of the two induction procedures used (assigned PRL, in which participants were trained to develop a PRL to the left or to the right of the scotoma) was chosen to match some of the studies simulating central vision loss in healthy participants (Kwon et al., 2013; Liu & Kwon, 2016) and to be used as a comparison for the more “ecological” annulus condition, in which participants were allowed to develop a PRL anywhere around the scotoma. We observed that roughly half of the participants in the assigned PRL group maintained their assigned PRL throughout the study, whereas the remaining half either did not develop a PRL or shifted its location to a different quadrant (Figure 1; see the Supplementary Material for individual graphs). It is important to mention that the literature on both clinical populations and healthy participants trained with artificial scotoma suggests that a PRL to either sides of the scotoma is less convenient than a PRL below or above it (Fine & Rubin, 1999; Nilsson, Frennesson, & Nilsson, 2003; Prahalad & Coates, 2020), thus a different training strategy could lead to better results in terms of changes in the oculomotor metrics.
Interestingly, a recent paper (Xie, Liu, & Yu, 2020) proposed an alternative strategy to PRL training, which consists of the development of a preferred retinal annulus (PRA), similar to our annulus induction procedure. In contrast to our induction procedure, which aimed at allowing the participants to spontaneously choose one retinal region within which to develop a PRL, Xie et al. (2020) trained healthy participants with a simulated, invisible central scotoma to develop 12 locations around the scotoma to be used as a saccade landing location depending on the proximity of the target. Results showed slower saccade latency but higher spatial accuracy in acquiring the target for the PRA group with respect to a standard PRL group (similar to our assigned PRL induction procedure). Indeed, additional information about these two training protocols could be acquired by applying the metrics from Maniglia et al. (2020) to the dataset of Xie et al. (2020).
A natural next step would be to apply these metrics to patients as they perform different tasks to gain a clearer understanding of the extent to which the details for the peripheral looking strategies found after experience with simulated scotomas resemble those found in individuals suffering from central vision loss, as well as how training interventions influence these oculomotor strategies.
In conclusion, experiencing simulated central vision loss while engaged in a visual task leads to changes in oculomotor behavior that can be captured when the eye tracking data are broken down into multiple metrics, each one addressing a different aspect of the eye movement strategies. Current eye movement analysis tends to examine only very general aspects such as fixation distribution. The data presented here suggest that oculomotor plasticity in response to deprivation of central vision manifests in different ways that our metrics can capture, allowing for a more complete description of profiles of compensation for loss of central vision. These profiles could be used both to study the time course of development of visual pathologies and to test potential treatment. In particular, by conducting assessment measurements on a number of visual tasks and correlating performance with oculomotor profiles of behavior, we will be able to better understand what type of compensatory eye movement strategies lead to better performance, which in turn can contribute to the development of individualized oculomotor training regimes.
Supplementary Material
Acknowledgments
Commercial relationships: none.
Corresponding author: Marcello Maniglia.
Email: mmanig@ucr.edu.
Address: Department of Psychology, University of California, Riverside, Riverside, CA, USA.
References
- Barraza-Bernal M. J., Rifai K., & Wahl S. (2017a). A preferred retinal location of fixation can be induced when systematic stimulus relocations are applied. Journal of Vision, 17(2):11, 1–13, 10.1167/17.2.11. [DOI] [PubMed] [Google Scholar]
- Barraza-Bernal M. J., Rifai K., & Wahl S. (2017b). Transfer of an induced preferred retinal locus of fixation to everyday life visual tasks. Journal of Vision, 17(14):2, 1–16, 10.1167/17.14.2. [DOI] [PubMed] [Google Scholar]
- Brainard D. H. (1997). The Psychophysics Toolbox. Spatial Vision, 10(4), 433–436. [PubMed] [Google Scholar]
- Cornelissen F. W., Peters E. M., & Palmer J. (2002). The EyeLink Toolbox: Eye tracking with MATLAB and the Psychophysics Toolbox. Behavior Research Methods, Instruments, & Computers, 34(4), 613–617. [DOI] [PubMed] [Google Scholar]
- Crossland M. D., Culham L. E., Kabanarou S. A., & Rubin G. S. (2005). Preferred retinal locus development in patients with macular disease. Ophthalmology, 112(9), 1579–1585. [DOI] [PubMed] [Google Scholar]
- Crossland M. D., Sims M., Galbraith R. F., & Rubin G. S. (2004). Evaluation of a new quantitative technique to assess the number and extent of preferred retinal loci in macular disease. Vision Research, 44(13), 1537–1546. [DOI] [PubMed] [Google Scholar]
- Cummings R. W., Whittaker S. G., Watson G. R., & Budd J. M. (1985). Scanning characters and reading with a central scotoma. Optometry and Vision Science, 62(12), 833–843. [DOI] [PubMed] [Google Scholar]
- Fine E. M., & Rubin G. S. (1999). Reading with simulated scotomas: Attending to the right is better than attending to the left. Vision Research, 39(5), 1039–1048. [DOI] [PubMed] [Google Scholar]
- Fletcher D. C., & Schuchard R. A. (1997). Preferred retinal loci relationship to macular scotomas in a low-vision population. Ophthalmology, 104(4), 632–638. [DOI] [PubMed] [Google Scholar]
- Guez J. E., Le Gargasson J. F., Rigaudiere F., & O'Regan J. K. (1993). Is there a systematic location for the pseudo-fovea in patients with central scotoma? Vision Research, 33(9), 1271–1279. [DOI] [PubMed] [Google Scholar]
- Jones P. R. (2019). A note on detecting statistical outliers in psychophysical data. Attention, Perception & Psychophysics, 81(5), 1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon M., Nandy A. S., & Tjan B. S. (2013). Rapid and persistent adaptability of human oculomotor control in response to simulated central vision loss. Current Biology, 23(17), 1663–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Q., & Chung S. (2020). Properties of the “preferred retinal locus” in response to asymmetrical progression of simulated central scotomas. Journal of Vision, 20(11), 1341, 10.1167/jov.20.11.1341. [DOI] [Google Scholar]
- Liu R., & Kwon M. (2016). Integrating oculomotor and perceptual training to induce a pseudofovea: A model system for studying central vision loss. Journal of Vision, 16(6):10, 1–21, 10.1167/16.6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z.-L., Lin Z., & Dosher B. A. (2016). Translating perceptual learning from the laboratory to applications. Trends in Cognitive Sciences, 20(8), 561–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniglia M., Pavan A., Sato G., Contemori G., Montemurro S., Battaglini L., & Casco C. (2016). Perceptual learning leads to long lasting visual improvement in patients with central vision loss. Restorative Neurology and Neuroscience, 34(5), 697–720. [DOI] [PubMed] [Google Scholar]
- Maniglia M., Visscher K. M., & Seitz A. R. (2020). A method to characterize compensatory oculomotor strategies following simulated central vision loss. Journal of Vision, 20(9):15, 1–18, 10.1167/jov.20.9.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson U. L. (1990). Visual rehabilitation with and without educational training in the use of optical aids and residual vision. A prospective study of patients with advanced age-related macular degeneration. Clinical Vision Science, 6(1), 3. [Google Scholar]
- Nilsson U. L., Frennesson C., & Nilsson S. E. G. (2003). Patients with AMD and a large absolute central scotoma can be trained successfully to use eccentric viewing, as demonstrated in a scanning laser ophthalmoscope. Vision Research, 43(16), 1777–1787. [DOI] [PubMed] [Google Scholar]
- Pelli D. G. (1997). The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision, 10(4), 437–442. [PubMed] [Google Scholar]
- Pelli D. G., Robson J. G., & Wilkins A. J. (1988). The design of a new letter chart for measuring contrast sensitivity. Clinical Vision Science, 2(3), 187–199. [Google Scholar]
- Plank T., Rosengarth K., Schmalhofer C., Goldhacker M., Brandl-Rühle S., & Greenlee M. W. (2014). Perceptual learning in patients with macular degeneration. Frontiers in Psychology, 5, 1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahalad K. S., & Coates D. R. (2020). Asymmetries of reading eye movements in simulated central vision loss. Vision Research, 171, 1–10. [DOI] [PubMed] [Google Scholar]
- Rosengarth K., Keck I., Brandl-Rühle S., Frolo J., Hufendiek K., Greenlee M. W., & Plank T. (2013). Functional and structural brain modifications induced by oculomotor training in patients with age-related macular degeneration. Frontiers in Psychology, 4, 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders D. R., & Woods R. L. (2014). Direct measurement of the system latency of gaze-contingent displays. Behavior Research Methods, 46(2), 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunness J. S., Applegate C. A., Haselwood D., & Rubin G. S. (1996). Fixation patterns and reading rates in eyes with central scotomas from advanced atrophic age-related macular degeneration and Stargardt disease. Ophthalmology, 103(9), 1458–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timberlake G. T., Mainster M. A., Peli E., Augliere R. A., Essock E. A., & Arend L. E. (1986). Reading with a macular scotoma. I. Retinal location of scotoma and fixation area. Investigative Ophthalmology and Visual Science, 27(7), 1137–1147. [PubMed] [Google Scholar]
- Van der Stigchel S., Bethlehem R. A. I., Klein B. P., Berendschot T. T. J. M., Nijboer T. C. W., & Dumoulin S. O. (2013). Macular degeneration affects eye movement behavior during visual search. Frontiers in Psychology, 4, 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Noorden G. K., & Mackensen G. (1962). Phenomenology of eccentric fixation. American Journal of Ophthalmology, 53, 642–660. [DOI] [PubMed] [Google Scholar]
- Walsh D. V., & Liu L. (2014). Adaptation to a simulated central scotoma during visual search training. Vision Research, 96, 75–86. [DOI] [PubMed] [Google Scholar]
- White J. M., & Bedell H. E. (1990). The oculomotor reference in humans with bilateral macular disease. Investigative Ophthalmology and Visual Science, 31(6), 1149–1161. [PubMed] [Google Scholar]
- Whittaker S. G., & Cummings R. W. (1990). Foveating saccades. Vision Research, 30(9), 1363–1366. [DOI] [PubMed] [Google Scholar]
- Wong W. L., Su X., Li X., Cheung C. M. G., Klein R., Cheng C.-Y., & Wong T. Y. (2014). Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. The Lancet Global Health, 2(2), e106–e116. [DOI] [PubMed] [Google Scholar]
- Xie X.-Y., Liu L., & Yu C. (2020). A new perceptual training strategy to improve vision impaired by central vision loss. Vision Research, 174, 69–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.