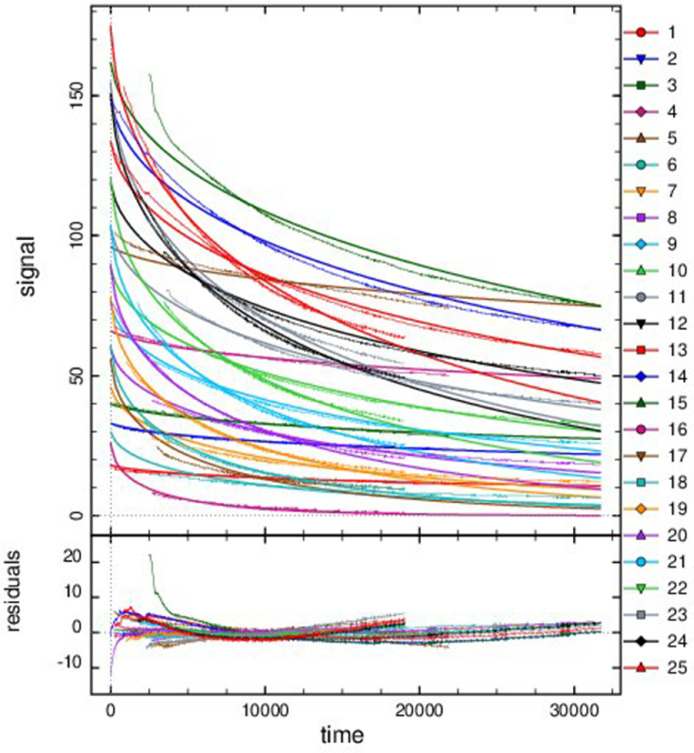
Fig. 5.
Progress curves of reactions catalyzed by AARCAp at different NADPH concentrations. The acetoacetyl-CoA concentration was fixed at 400 mM. Time scale is in seconds. The thick lines represent the global fitting, while thinner lines represent the experimental data. Best fit curves were obtained with a competitive product inhibition model. The initial concentrations of NADPH and enzyme were: (1) NADPH = 18 mM, Enzyme = 0.05 mM. (2) NADPH = 33 mM, Enzyme = 0.05 mM. (3) NADPH = 40 mM, Enzyme = 0.05 mM. (4) NADPH = 66 mM, Enzyme = 0.05 mM. (5) NADPH = 96 mM, Enzyme = 0.05 mM. (6) NADPH = 30 mM, Enzyme = 0.5 mM. (7) NADPH = 46 mM, Enzyme = 0.5 mM. (8) NADPH = 60 mM, Enzyme = 0.5 mM. (9) NADPH = 76 mM, Enzyme = 0.5 mM. (10) NADPH = 89 mM, Enzyme = 0.5 mM. (11) NADPH = 103 mM, Enzyme = 0.5 mM. (12) NADPH = 119 mM, Enzyme = 0.5 mM. (13) NADPH = 134 mM, Enzyme = 0.5 mM. (14) NADPH = 149 mM, Enzyme = 0.5 mM. (15) NADPH = 162 mM, Enzyme = 0.5 mM. (16) NADPH = 26 mM, Enzyme = 1.5 mM. (17) NADPH = 56 mM, Enzyme = 1.5 mM. (18) NADPH = 61 mM, Enzyme = 1.5 mM. (19) NADPH = 78 mM, Enzyme = 1.5 mM. (20) NADPH = 90 mM, Enzyme = 1.5 mM. (21) NADPH = 104 mM, Enzyme = 1.5 mM. (22) NADPH = 121 mM, Enzyme = 1.5 mM. (23) NADPH = 155 mM, Enzyme = 1.5 mM. (24) NADPH = 151 mM, Enzyme = 1.5 mM. (25) NADPH = 175 mM, Enzyme = 1.5 mM.